The most frequently used method for post-staining is a twostep procedure of staining with uranyl acetate (UA), followed by lead citrate. Uranyl acetate is used as an aqueous or alcoholic solution with a pH for the saturated solution in the range of 3.5 to 4.0. The addition of alcohol, especially methanol, increases the solubility (Hayat, 2000). Uranyl acetate strongly stains proteins as well as nucleic acids and phospholipids. When applied after the uranyl acetate staining, lead citrate (prepared according to Reynolds, 1963) will increase this contrast (Dykstra, 1992). Staining can be performed either manually or automatically, both techniques have their advantages. For manual staining, a grid is floated, sectionside down, on a drop of a uranyl acetate solution for 10 minutes. After blotting off the stain, the grid is rinsed thoroughly with water to remove any residual unbound stain. This first step is followed by 5 min. lead citrate staining, following the same procedure. The consumption of reagents is minimal, whereas the effort is relatively high.
Alternatively, poststaining can be automated. This ensures increased reproducibility and time saving, though the amount of reagents used is higher. Using the automated contrasting device EM AC20 (Leica Microsystems, Vienna) allows for simultaneous staining of up to 20 grids per run with no effort and a guarantee for safety, both for the environment and the user. Although UA is an excellent and well characterized stain, replacements are sought for, for several reasons.
When it needs to be handled as a powder, it is very toxic and carcinogenic if inhaled. Furthermore, also depleted uranyl acetate is considered a radioactive material, and hence subject to relevant regulations. Therefore, UA requires adequate storage and careful handling, which in turn increases cost for shipping and waste disposal. To minimize contact, the automated version with the AC20 is preferred by many users, in particular as readily prepared solutions are available, handling of solid UA can be avoided.
Two reagents described in literature as replacements for UA caught our attention: oolong tea extract (OTE) and Platinum Blue. Only very little data is published about them and the methods are not well known in the EM community. Therefore, we have tested both with manual contrasting and for the first time with the Leica EM AC20 instrument.
To allow a direct comparison of results from the different post-staining techniques, the same sample was used for all tests: Liver tissue freshly dissected from mice was fixed with 2.5 % glutaraldehyde in 100 mmol/l Soerensen phosphate buffer and post-fixed with 2.0 % osmium tetroxide. Pieces of tissue were dehydrated and embedded in Agar 100 epoxy resin and sectioned to a nominal thickness of 70 nm. Poststaining was performed as described below. Besides contrasting efficiency and comparability of the results with UA, a number of other important parameters were assessed.
Oolong tea extract
OTE was described as post-stain to electron microscopy by Sato et al. (2003), and used in a small number of studies (Sato et al., 2008; Miller and Simakova, 2010). According to Rumpler et al. (2001), OTE is a type of half-fermented tea and produced as a foodstuff. Therefore it was assumed to be harmless for health and environment, even though the supplier failed to produce a material safety data sheet. It can be purchased from Ted Pella (http://www.tedpella.com), and is delivered as a powder. According to Sato et al. (2003), the polyphenolic components in OTE react with peptide bonds. A reaction with OTE and lead citrate in return, leads to an enhancement of the contrast.
After tests with different OTE concentrations, 0.2 % OTE dissolved in boiling ddH2O was used for further experiments, as already proposed by Sato et al. (2003). Compared to conventional staining with uranyl acetate, the manual application of OTE and the subsequent staining step with Reynold’s lead citrate was more time consuming (Table 1). Optimal results on the Leica EM AC20 were obtained also using an extended program at room temperature (Table 2).
Tab. 1: Details of manual staining procedures
| Stain 1 | Time | Washing | Stain 2 | Time | Washing |
|---|---|---|---|---|---|
| UA | 10 min | 2 min ddH2O | Lead citrate | 5 min | 2 min ddH2O |
| OTE | 25 min | 6 min ddH2O | Lead citrate | 5 min | 2 min ddH2O |
| Pt Blue | 10 min | 2 min ddH2O | Lead citrate | 5 min | 2 min ddH2O |
Tab. 2: Automated staining procedures of the Leica EM AC20. The conditions for UA and lead citrate are suggested by Leica and can be found in the user's manual of the staining device.
| Stain 1 | Time | Washing | Stain 2 | Time | Washing |
|---|---|---|---|---|---|
| UA | 30 min | 2 min 20 sec ddH2O | Lead citrate | 7 min | 2 min 20 sec ddH2O |
| OTE | 40 min | 5 min ddH2O | Lead citrate | 7 min | 2 min ddH2O |
| Pt Blue | 30 min | 2 min 20 sec ddH2O | Lead citrate | 7 min | 2 min 20 sec ddH2O |
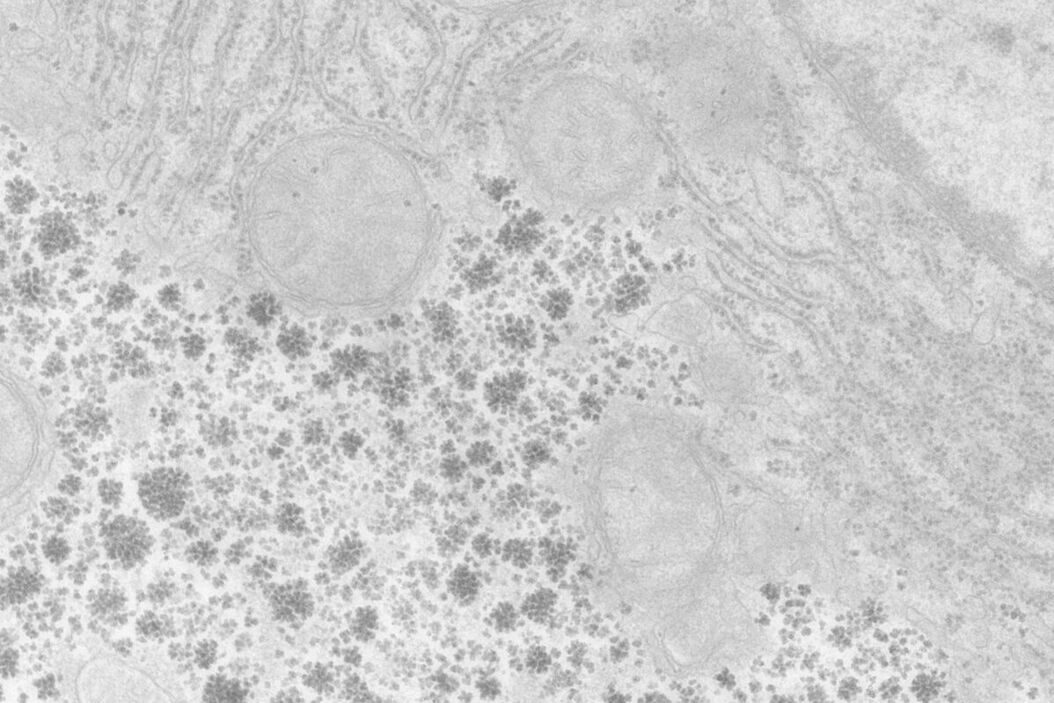
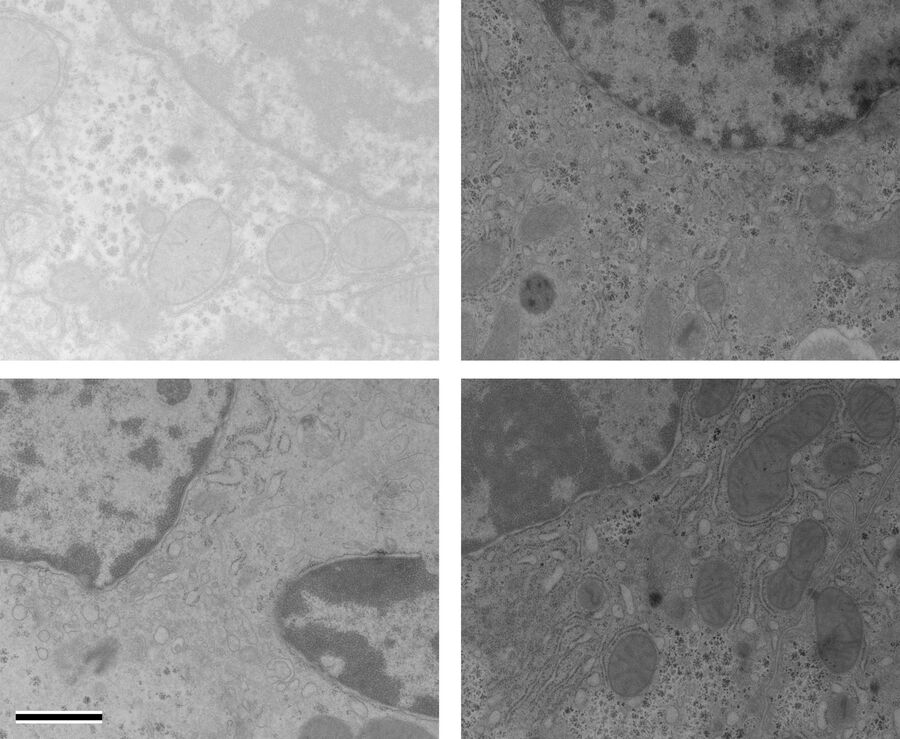
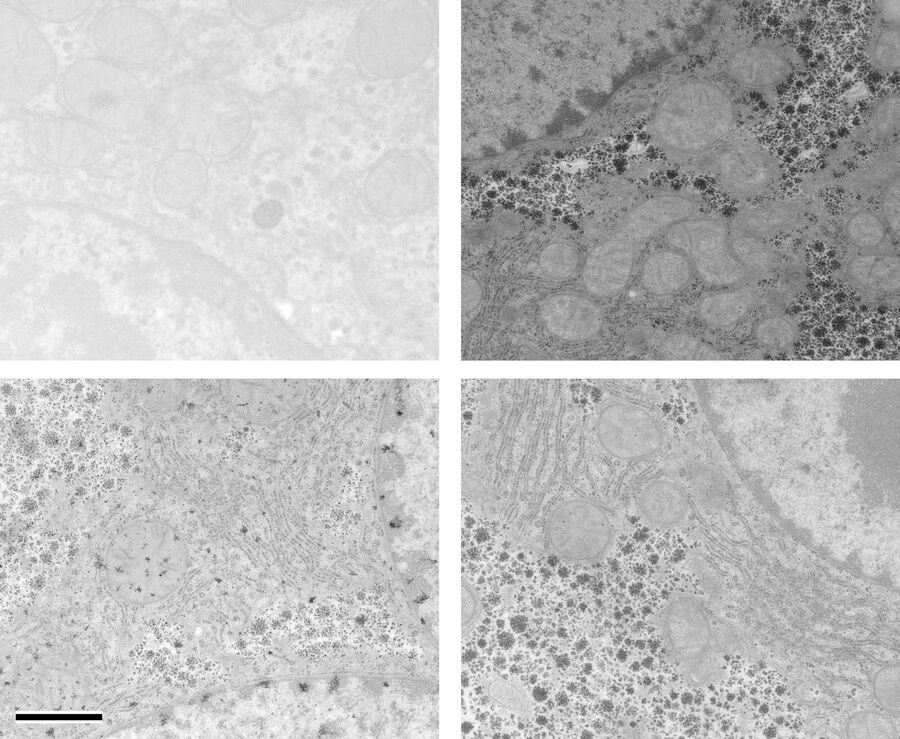
At a concentration of 0.2 % OTE both manually as well as automatically stained samples show an increase in contrast even though it was clearly lower than with UA (Figures 1 and 2). In our hands, contamination was observed more frequently than with UA, which mainly constituted a problem at lower magnifi cation. Despite extended washing steps, both with manual staining or the Leica EM AC20, this problem could not be eliminated.
Platinum Blue
Platinum Blue (Pt Blue) was used as an alternative to uranyl acetate in thin section post-staining by Inaga et al. (2007; 2009). This compound is a product of the reaction of cisdichlordiamineplatinum (II) with thymidine (Inaga et al., 2007). The reagent can be ordered directly from the Japanese producer Nisshin (http://nisshin-em.co.jp). Pt Blue is a hazardous material, which may cause eye irritation, cancer and effects on fertility as well as severe disorders of the bone marrow, kidneys and the nervous system (MSDS Nisshin). The commercially available 6 % stock solution has been found to give good results at a dilution of 1:100 for the manual staining and 1:200 for the automated procedure. The incubation times for the manual staining method were the same as for UA staining (cf. Table 1).
For staining using the Leica EM AC20, the step for Pt Blue were extended to 30 min, using the identical conditions as for UA. The grids were then rinsed with distilled water, stained with lead citrate, and washed as described in Table 2. Figures 1 and 2 illustrate that all organelles show good contrast and that the results are comparable in quality to pictures taken from sections stained with UA. Differences can be found in a more intensely stained mitochondrial matrix and a higher contrast of glycogen granules. The ribosomes appear weaker stained as compared to UA.
Conclusion
OTE and Pt Blue were assessed as substitutes for UA in section post-staining for electron microscopy with regard to contrasting effi ciency, toxicity, handling, and price. Manual as well as automatic staining procedures with the Leica EM AC20 were tested. No en bloc staining was performed. Furthermore, resins other than Agar 100 or different section thicknesses may lead to different results.
Both the rather weak contrast obtained with OTE as well as the contamination observed on the specimen were not convincing with manual staining and the Leica EM AC20. Pt Blue delivered clearly better results with both approaches. However, slight differences in contrasting properties as compared to UA have to be taken into account for interpretation of micrographs (Yamaguchi et al., 2010). The quality of results that can be obtained from automatic staining is comparable with the manual procedure for both OTE and Platinum Blue.
Regarding toxicity, both reagents tested as substitutes of UA have the advantage of not being radioactive. Furthermore, working with health-damaging inhalable powder can be avoided. Being a food product it can be presumed that OTE is non-hazardous. In contrast, Pt Blue is toxic but as it is delivered as a stock solution further risky handling can be reduced to a minimum. The utilization of an automatic staining device such as the Leica EM AC20 can help to further minimize contact with toxic reagents.
Staining with UA and Pt Blue is comparable in time and labor, whereas contrasting with OTE is more time consuming without delivering as satisfactory results. As with the Leica EM AC20, it is possible to stain up to 20 grids at once and all steps are carried out automatically, this becomes less of a disadvantage of OTE. From the economic point of view, OTE was by far the cheapest product. The price per grid at the used concentration of 0.2 % was signifi cantly lower than for Pt Blue (at a dilution of 1:100) and the 2.0 % UA solution.
Summing up, electron microscopists in need of a replacement for UA for post-staining of sections have the choice between one reagent at a very cheap price and minimal risk with moderate results – Oolong tea extract – and another one, that delivers very convincing results, but at higher cost and not without safety risks – Platinum Blue. The experiments here have shown that this is applicable for both manual as well as automated staining with the
Leica EM AC20.
Acknowledgements
The authors would like to thank Yanli Tong (Leica Microsystems, Shanghai) for assistance with acquiring reagents and Jean Trichereau (IMBA, Vienna) for providing samples. The work of N.F., M.B. and G.P.R. was supported by the City of Vienna/Zentrum fuer Innovation und Technologie through the Spot of Excellence grant "Center of Molecular and Cellular
Nanostructure".
References
- MSDS Nisshin: http://nisshin-em.co.jp/home/msds/index.html.
- Dykstra MJ: Biological Electron Microscopy: Theory, Techniques, and Troubleshooting. Plenum Press, New York (1992).
- Flegler SL, Heckman JW Jr, Klomparens KL: Scanning and Transmission Electron Microscopy: An Introduction. W.H. Freeman and Company, New York (1993).
- Hayat MA: Principles and Techniques of Electron Microscopy: Biological Applications. 4th ed. Cambridge University Press, Cambridge (2000).
- Inaga S, Hirashima S, Tanaka K, Katsumoto T, Kameie T, Nakane H, Naguro T: Low vacuum scanning electron microscopy for paraffin sections utilizing the differential stainability of cells and tissues with platinum blue. Arch Histol Cytol 72:2 (2009) 101–106.
- Inaga S, Katsumoto T, Tanaka K, Kameie T, Nakane H, Naguro T: Platinum blue as an alternative to uranyl acetate for staining in transmission electron microscopy. Arch Histol Cytol 70:1 (2007) 43–49.
- Miller AA., Simakova AV: Application of Method of OTE Staining of Ultrathin Sections Based on Example of Microsporidia (Protozoa: Microsporidia). Cell and Tissue Biology 4:1 (2010) 109–115.
- Reynolds ES: The Use of Lead Citrate at High pH as an Electron Opaque Stain in Electron Microscopy. Journal of Cell Biology 17 (1963) 208–212.
- Sato S, Adachi A, Sasaki Y, Ghazizadeh M: Oolong tea extract as a substitute for uranyl acetate in staining of ultrathin sections. Journal of Microscopy 229:Pt 1 (2008) 17–20.
- Sato S, Sasaki Y, Adachi A, Dai W, Liu XL, Namimatsu S: Use of oolong tea extract (OTE) for elastin staining and enhancement in ultrathin sections. Med Electron Microsc 36:3 (2003) 179–182.
- Rumpler W, Seale J, Clevidence B, Judd J, Wiley E, Yamamoto S, Komatsu T, Sawaki T, Ishikura Y, Hosoda K: Oolong Tea Increases Metabolic Rate and Fat Oxidation, in: Men J Nutr 131 (2001) 2848–2852.
- Yamaguchi K, Suzuki K, Tanaka K: Examination of electron stains as a substitute for uranyl acetate for the ultrathin sections of bacterial cells. Journal of Electron Microscopy (Tokyo) 59:2 (2010) 113–118.
Note added in proof: Readers interested in replacing uranyl acetate are also referred to Nakakoshi M, Nishioka H and Katayama E. New versatile staining reagents for biological transmission electron microscopy that substitute for uranyl acetate. Journal of Electron Microscopy 60:6 (2011) 401–407.
Related Articles
-
How Marine Microorganism Analysis can be Improved with High-pressure Freezing
In this application example we showcase the use of EM-Sample preparation with high pressure…
Aug 07, 2023Read article -

Advancing Cellular Ultrastructure Research
Freeze-fracture and freeze-etching are useful tools for studying flexible membrane-associated…
Oct 04, 2021Read article -
Targeting Active Recycling Nuclear Pore Complexes using Cryo Confocal Microscopy
In this article, how cryo light microscopy and, in particular cryo confocal microscopy, is used to…
Mar 31, 2021Read article