Developmental Biology Images taken with THUNDER Imagers
See how Leica Microsystems instruments can help imaging development, regeneration, reproduction, growth, differentiation, and metamorphosis of different organisms. Organisms often studied include mice, rats, chickens, C. elegans (roundworms), Drosophila (fruit flies), pollinating flowers, zebrafish, and humans.
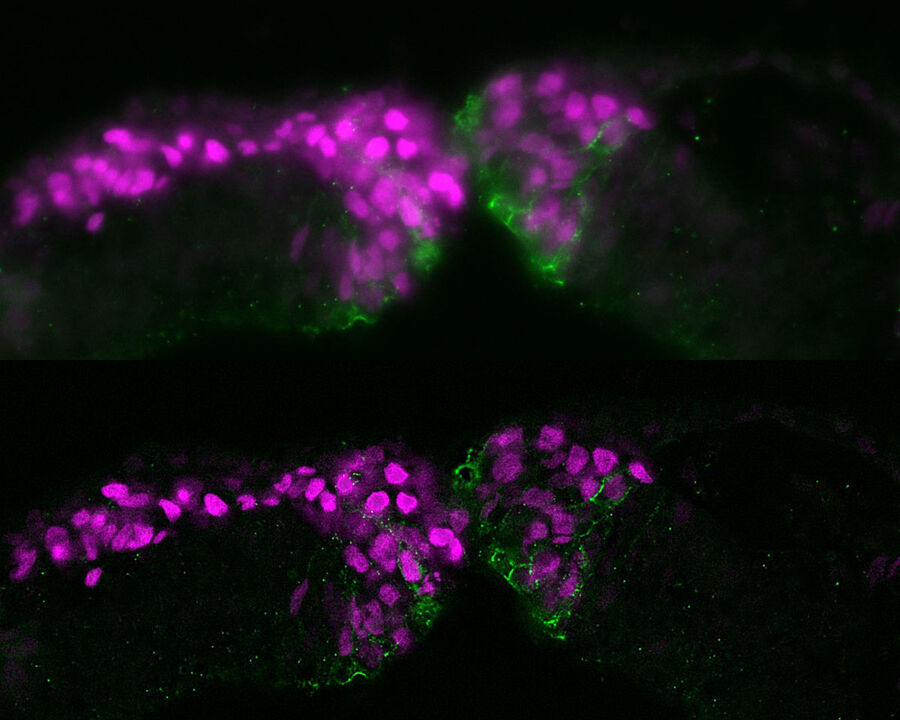
Neural crest (NC) embryonic cell population
The neural crest (NC) is an embryonic cell population with remarkable multipotency and migratory ability enabling it to contribute to diverse organ systems, including the craniofacial skeleton and the peripheral nervous system. The Bronner lab at the California Institute of Technology is interested in understanding the mechanisms underlying the specification, migration, and differentiation of neural crest cells in multiple vertebrate embryos.
Pictured is a cross-section of a chicken embryo at the level of the midbrain showing neural crest cells (magenta) as they undergo epithelial-to-mesenchymal transition and begin to migrate. Neural crest cells normally downregulate levels of the cell adhesion molecule Cadherin-6B (green) and then begin to migrate laterally (left side of image). In neural crest cells in which epithelial-to-mesenchymal transition is experimentally blocked (right side of image), Cadherin-6B levels remain high and the neural crest fails to leave the neural tube epithelium.
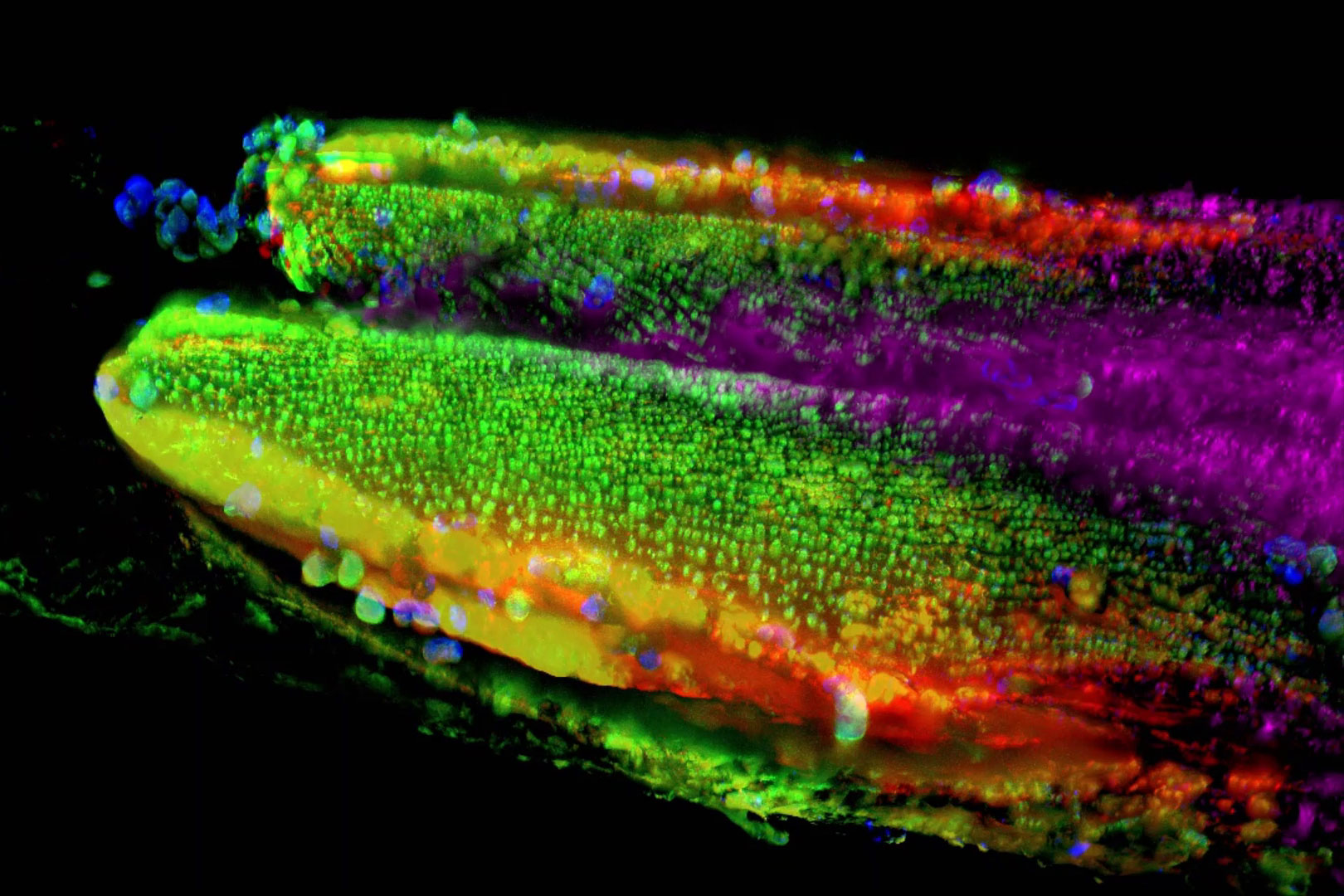
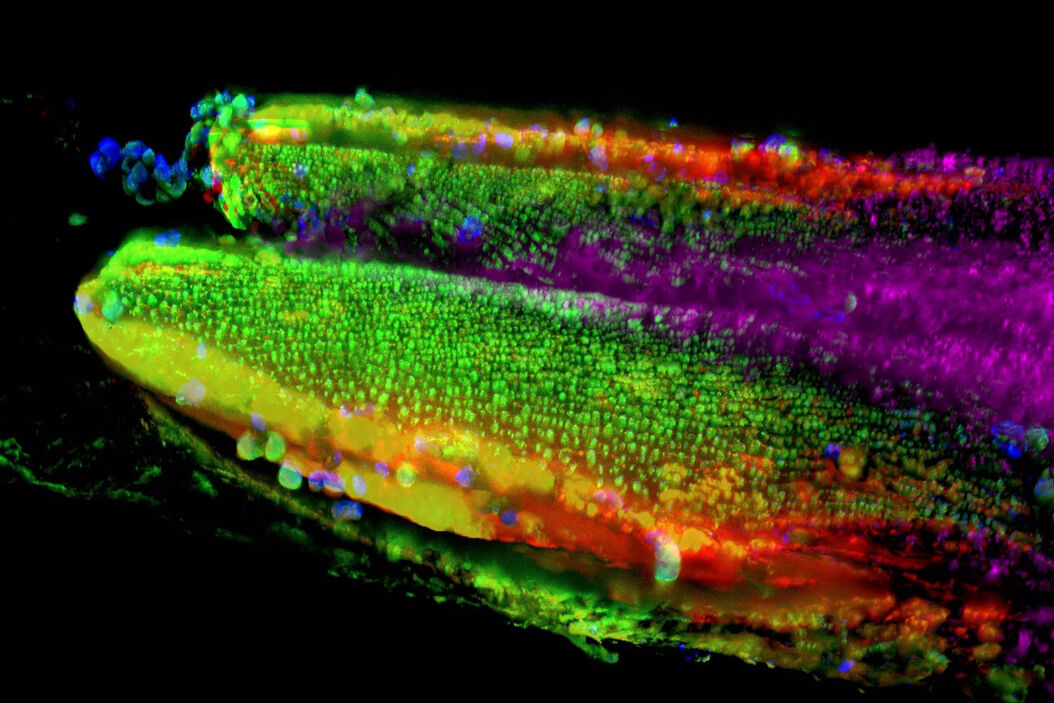
Autophagy and age-related pathologies - C. elegans
Understanding the interplay between the accumulation of DNA damage and age-related pathologies such as neurodegeneration is important. Autophagy is a highly regulated and dynamic process that clears cells of unwanted organelles, and protein aggregates. Double layer membranes called autophagosomes engulf cargo that is destined for the degradation process. Autophagosomes fuse to lysosomes forming autolysosomes that degrade autophagosome-delivered cargo. Autophagy is compromised with age and is known to be dysfunctional in several age-related pathologies.
Mouse embryo
Transgenic, perinatal mouse heart
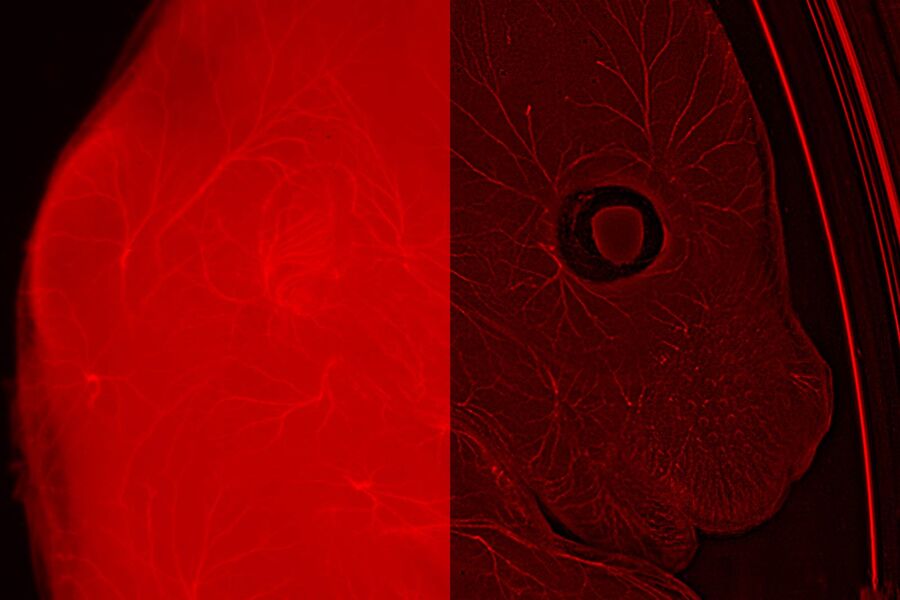
Using the developing mouse embryo as a model, researchers in Michelle Tallquist’s laboratory at the University of Hawaii at Manoa aim to increase our understanding of human birth defects and diseases. During embryonic development, cell movement, proliferation, and differentiation must be tightly orchestrated in order to generate healthy progeny. The Tallquist group primarily focuses on the function of platelet derived growth factor receptors, PDGFRα and PDGFRβ, in mammalian development and disease.
A major challenge in developmental biology is tracking proteins or cells of interest in vivo. Moreover, organs like the heart tend to be very autofluorescent, which obscures visualization of fluorescently-tagged proteins. Using the THUNDER Imager 3D Cell Culture, the high background fluorescence of a perinatal mouse heart was significantly reduced, allowing for easier identification of PDGFRα-positive cells.
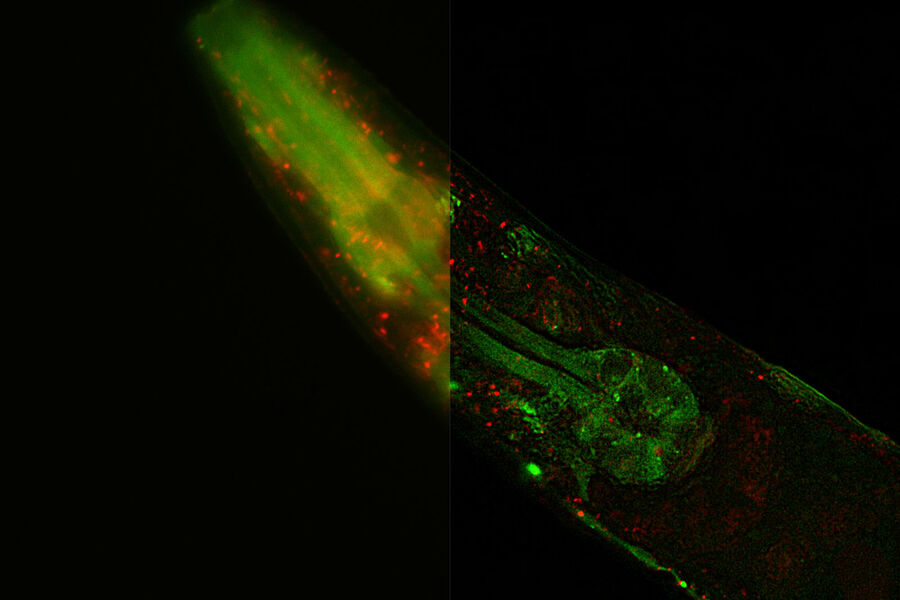
C. elegans. Transgenic GFP
Zebrafish embryo
Drosophila embryo
Drosophila Follicles
Adipose Tissue Development and Expansion - Sharp Imaging of Whole Mount Fat Specimens
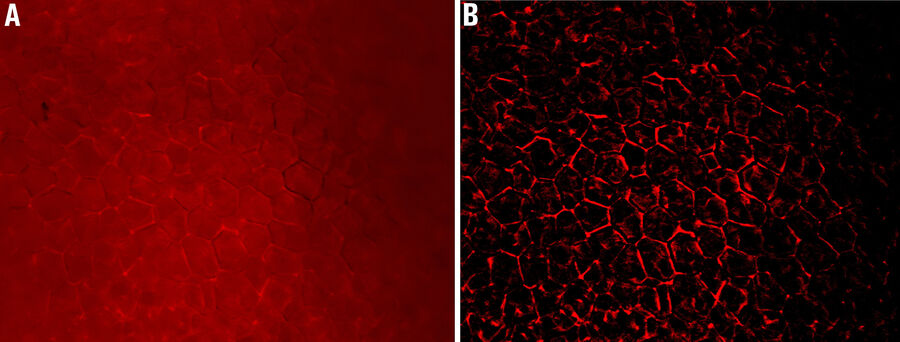
Pollen Flower
Adult mouse ovary - Germ cell development
The research group of Dr. Diana Laird at the University of California, San Francisco, focuses on three interrelated questions: do all developing germ cells have equal potential to give rise to functional eggs or sperm; how do environmental inputs affect germ cells during development, and what is the role of germ cells in ovarian and systemic aging? Using mouse models and human cells, they explore these questions in the real-world contexts of prenatal exposures to endocrine-disrupting chemicals and psychosocial stress as well as genetic causes of infertility such as Fragile X Primary Ovarian Insufficiency.
Large Volume Computational Clearing
One research area in the Laird Lab is aging and ovarian reserve. By using the mouse ovary as a model, postdoctoral fellow Dr. Bikem Soygur is studying germ cell maintenance and aging at various timepoints, from embryonic development through adulthood. One challenge is that the adult ovary is difficult to image, even if it is cleared by optical refractive index matching methods. A key marker Dr. Soygur uses is an antibody against NOBOX (newborn ovary homeobox gene), which is an oocyte-specific homeobox gene that plays a critical role in early folliculogenesis and represents a candidate gene for nonsyndromic ovarian failure.
Using the THUNDER Imager 3D Cell Culture, one can image the whole mouse ovary in minutes, then perform Large Volume Computational Clearing (LVCC) to obtain higher contrast images without the haze of traditional widefield images.
Related Articles
-
What are the Challenges in Neuroscience Microscopy?
eBook outlining the visualization of the nervous system using different types of microscopy…
Jun 14, 2023Read article -
Central Nervous System (CNS) Development and Activity in Organisms
This article shows how studying central nervous system (CNS) development in Drosophila-melanogaster…
May 12, 2023Read article -
Going Beyond Deconvolution
Widefield fluorescence microscopy is often used to visualize structures in life science specimens…
Mar 22, 2023Read article