Programming the sequence
Two electron guns are being used in the course of this preparation, both of which need to be degassed in order to avoid a considerable degradation of the vacuum due to heating in the moment the actual evaporation starts. Instead of two subsequent degas/pump/evaporate cycles for Pt/C and C, it is preferred to degas both guns first, then pump for considerable time to reach a good process vacuum required to evaporate both materials. An automated sequence implementing this approach is being described here. The Pt/C gun is mounted at the right port, the C gun at the left side.
Materials
- A material "Platinum degas" was defined using Pt standard settings, 50 W degassing power, and 120 s degassing.
- A material "Carbon degas" was defined using C standard settings, 75 W degassing power, and 300 s degassing.
- Two materials "Platinum no degas" and "Carbon no degas" were defined with the same properties as the original materials, but degassing was deactivated.
Processes
- A process "Degassing Pt/C" designed to degas the Pt/C gun only was programmed using the material "Platinum degas". The process was set to start at 1.0 × 10–4 mbar, with an evaporation power of low 30 W, and a target thickness of 0.0 nm to terminate immediately after opening the shutter. Setting the stage position was hence obsolete.
- A process "Degassing C" designed to degas the C gun only was programmed using the material "Carbon degas". The process was set to start at 1.0 × 10–4 mbar, with an evaporation power of low 30 W, and a target thickness of 0.0 nm to terminate immediately after opening the shutter. Setting the stage position was hence obsolete.
- A process "Shadowing Pt/C" was programmed using the following settings: Material "Platinum no degas", 100 W target power, 2.0 nm target thickness, vacuum < 2.5 × 10–5 mbar, stage at –54° tilt (corresponding to 5° relative to the gun), maximum working distance, and rotation 5 (maximum).
- A process "Backing C" was programmed using the following settings: Material "Carbon no degas", 120 W targat power, 6.0 nm target thickness, vacuum < 2.5 × 10–5 mbar, stage at 20° tilt (relative to horizontal), maximum working distance, and rotation 5 (maximum).
Sequence
- A sequence consisting of "Degassing Pt/C", "Degassing C", "Shadowing Pt/C", and "Backing C" was prepared.
Preparation
Prior to the experiment, both the Pt/C and the C gun were cleaned thoroughly, the targets and the filaments were set up according to the manual. The Pt/C gun was mounted at the right port of the vacuum chamber, the C gun at the left side. The instrument was set up with the LARS (low angle rotary shadowing) stage and the quartz crystal at the lateral position.
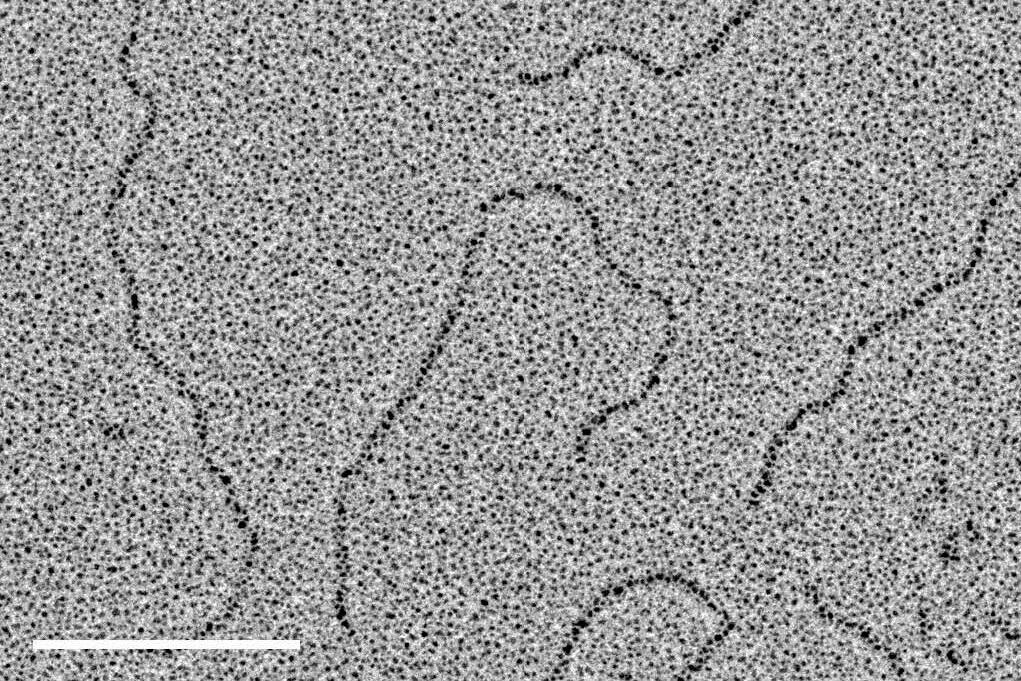
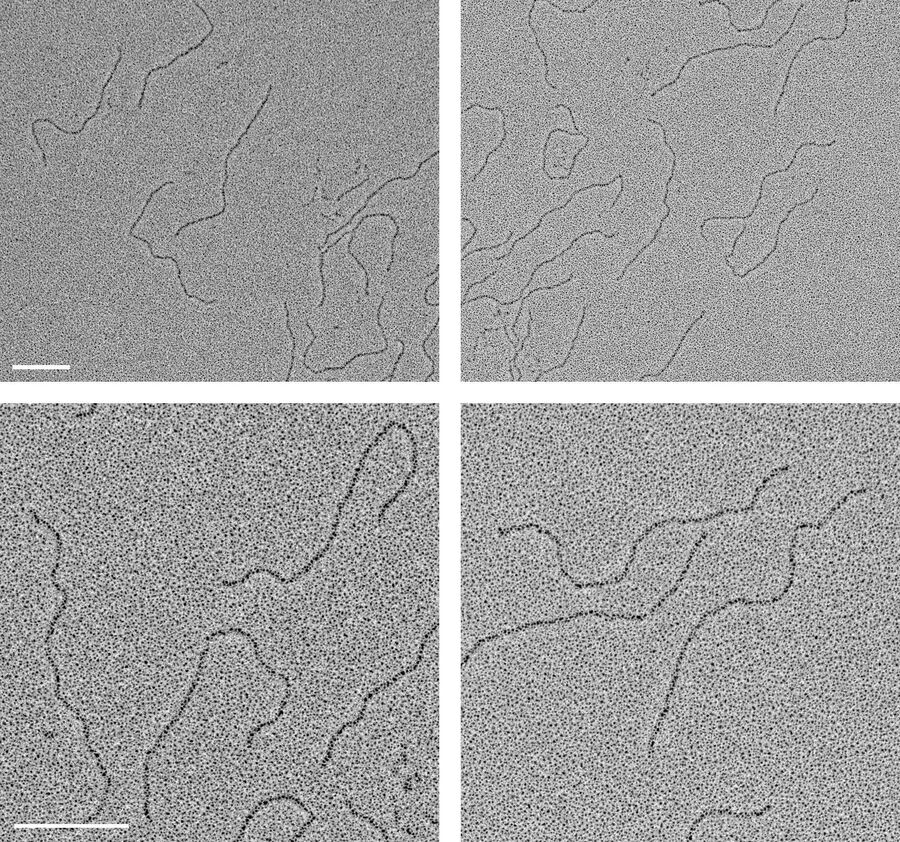
130 µg/ml dsDNA with a length of 1,010 basepairs (corresponding to 343 nm) were diluted 1:20 in spraying buffer (100 mmol/l ammonium acetate, 30 % glycerol, pH 7.6). Small droplets were sprayed with a spraying device as described in [1] onto the freshly cleaved surface of mica chips approximately 2 × 2 mm in size and mounted with double-sided sticky tape on a 50 mm round filter paper.
After spraying, the samples on the filter paper were transferred immediately to the readily prepared Leica EM ACE600 and evacuated. The sequence as outlined above was executed, the chamber was automatically vented upon completion.
The replicas on the filter paper were removed from the vacuum chamber and transferred to a humid cham-ber, made from plastic dishes, Parafilm (to keep samples from getting wet), and wet filter paper. To facilitate detachment of the replicas, they were incubated in an oven at 45 °C for 30 minutes.
Subsequently, the replicas were floated off on the surface of distilled water in a white dish, picked up with an un-filmed 400 mesh Cu grid, transferred to another dish with ddH2O for removal of all residual organic material (glycerol) and picked up with another grid. Excess water was blotted from the side and from the tips of the forceps with filter paper, samples were air dried.
The air dried replicas were observed in a transmission electron microscope at 80 kV, regions of interest could be located via crystals of residual salt forming at the center of the dried droplets as described in [1]. Images were recorded with a CCD camera at a pixel size of 0.373 nm/pixel.
Acknowledgements
The author thanks Karel Riha's group at the Gregor Mendel Institute, Vienna, for providing the sample and the team at the Electron Microscopy Facility of the Campus Science Support Facilities GmbH (www.csf.ac.at/em), Vienna, for their assistance.
Reference
- Aebi U., and Baschong W: Glycerol Spraying/Low-Angle Rotary Metal Shadowing, in: Celis JE (ed.): Cell Biology – A Laboratory Handbook, third edition, 3: 241–46 (2006).
Related Articles
-
How Marine Microorganism Analysis can be Improved with High-pressure Freezing
In this application example we showcase the use of EM-Sample preparation with high pressure…
Aug 07, 2023Read article -

Advancing Cellular Ultrastructure Research
Freeze-fracture and freeze-etching are useful tools for studying flexible membrane-associated…
Oct 04, 2021Read article -
Targeting Active Recycling Nuclear Pore Complexes using Cryo Confocal Microscopy
In this article, how cryo light microscopy and, in particular cryo confocal microscopy, is used to…
Mar 31, 2021Read article
Related Pages
-

Visualization of DNA Molecules
Precise low angle rotary shadowing with heavy metals (like platinum) can be used in transmission…
Visit related page