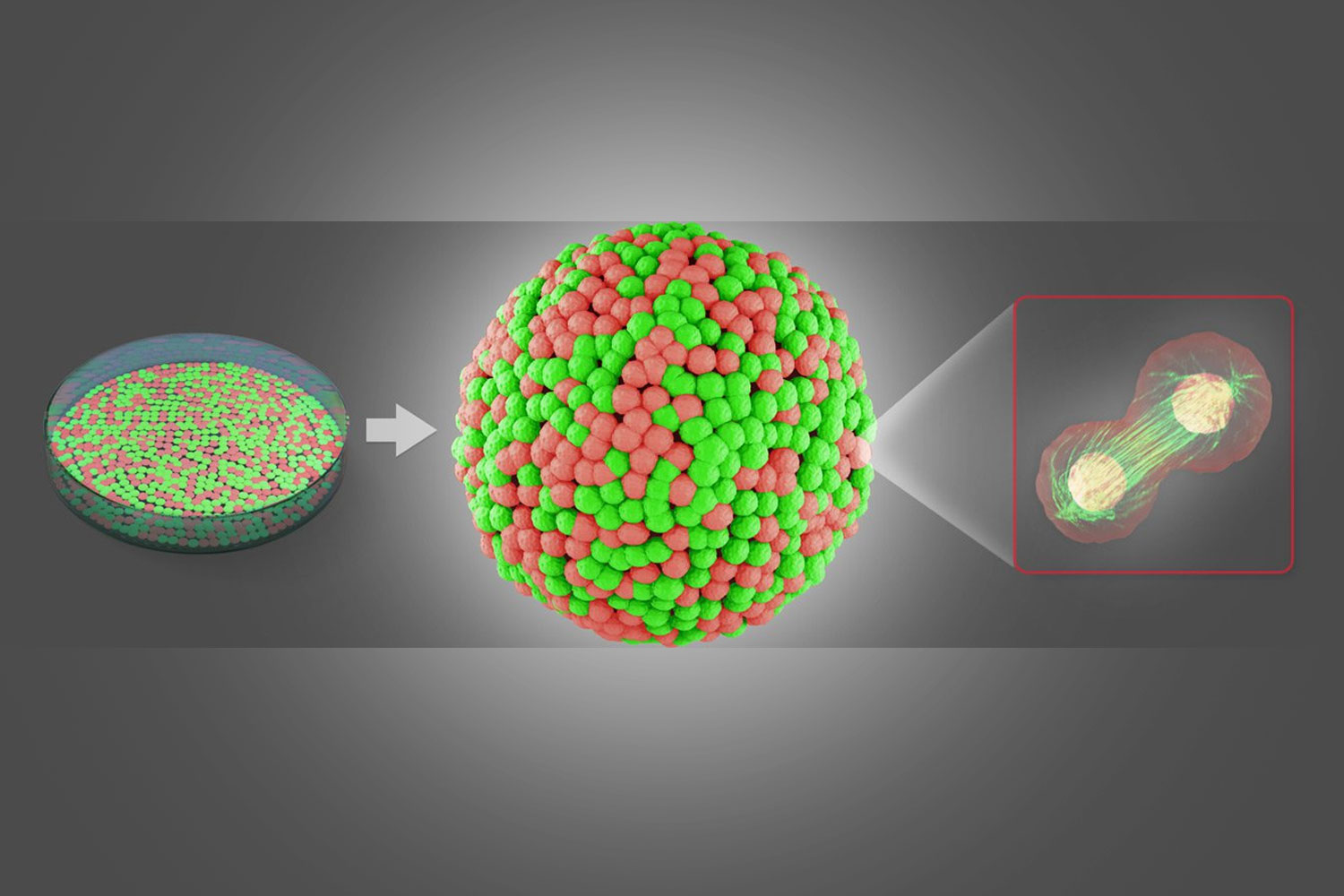
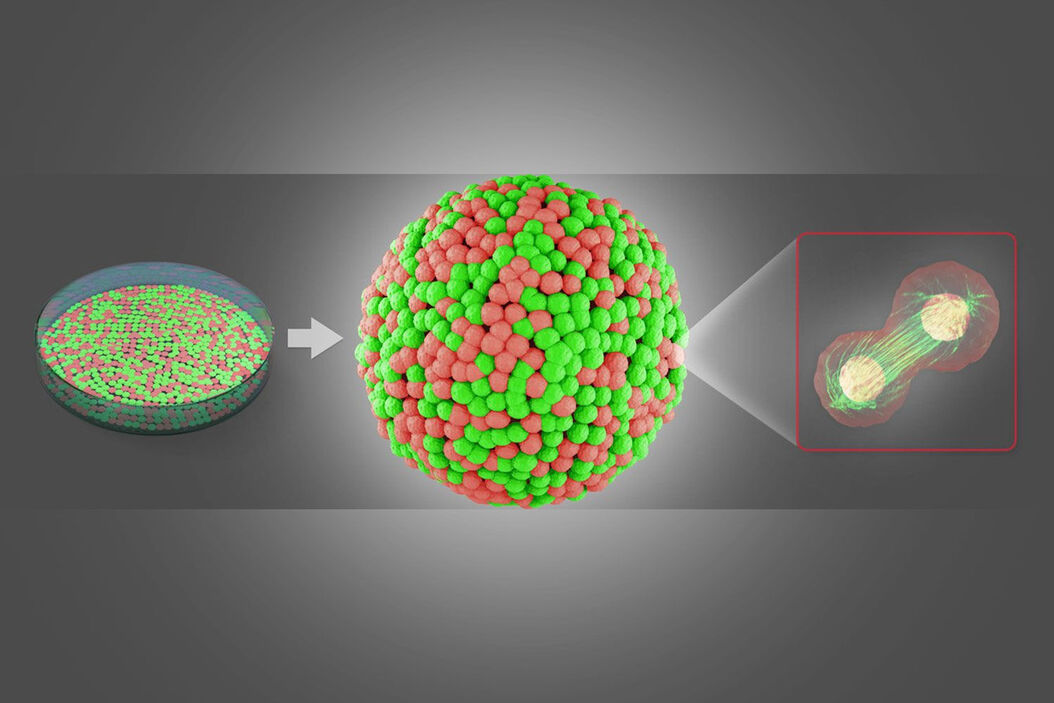
Other fields of research: Neuroscience
In neuroscience, amongst other advances, an organoid model for Zika virus has been developed to study the brain. These organoids are ideal in vitro models of development, disease pathogenesis, and drug screening.
Further reading on 3D Biology
Ishiguro et al., Cancer Science, 2017: Tumor-derived spheroids: Relevance to cancer stem cells and clinical applications.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5378268/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5378268/Halfter and Mayer, Biotechnol J, 2017: Bringing 3D tumor models to the clinic- predictive value for personalized medicine.
https://www.ncbi.nlm.nih.gov/pubmed/28098436
Sato and Clevers, Cell, 2015: Snap Shot: Growing Organoids from Stem Cells
https://www.ncbi.nlm.nih.gov/pubmed/26091044
Challenge for conventional microscopy
A constantly ascending number of culturing methods allows cancer cells to be grown as 3D spheres. These cellular spheroids mimic the properties of solid tumors and are well suited to model cancer development with physiological relevance. The complex 3D structure and volumetric size represents a challenge in conventional light microscopy. This can be overcome by light sheet-based microscopy, which allows tracking of sub-cellular changes in large samples within reasonable time.
Amplify your results
The investigation of cellular spheroids with the Leica SP8 Digital LightSheet (DLS) microscope reveals meaningful results of cellular and molecular processes in standard petri dishes and is very well suited for cancer research.
Spheroid imaging workflow with multi-position experiments
Leica Microsystems’ Digital LightSheet module (DLS) can be integrated in the vertical axis of any inverted confocal Leica TCS SP8 microscope.
- Save time by easy sample preparation in standard petri dishes.
- Imaging of multiple spheroids in one experiment allows for highly productive long-term observation.
3D Cell Biology, Spheroids, Organoids
In the past decade, the application of 3D cell cultures has grown significantly and has developed into a robust and reliable cellular model. More and more scientists are convinced that 3D cell cultures will soon replace conventional monolayer cell cultures.
Recent examples of 3D cell culture models elucidate new insights into colorectal cancer (CRC) and its complex underlying development mechanisms. In this case organoids show numerous valuable applications in disease research as well as in regenerative and personalized medicine.
- 3D Spheroid Culture Technology Trends 2016. HTStec 2016
- Cristobal et al., Cell Reports, 2017
- Dang et al., Cell Stem Cell, 2016
- Shimokawa et al., Nature, 2017
3D Cell Biology Workflow - Steps
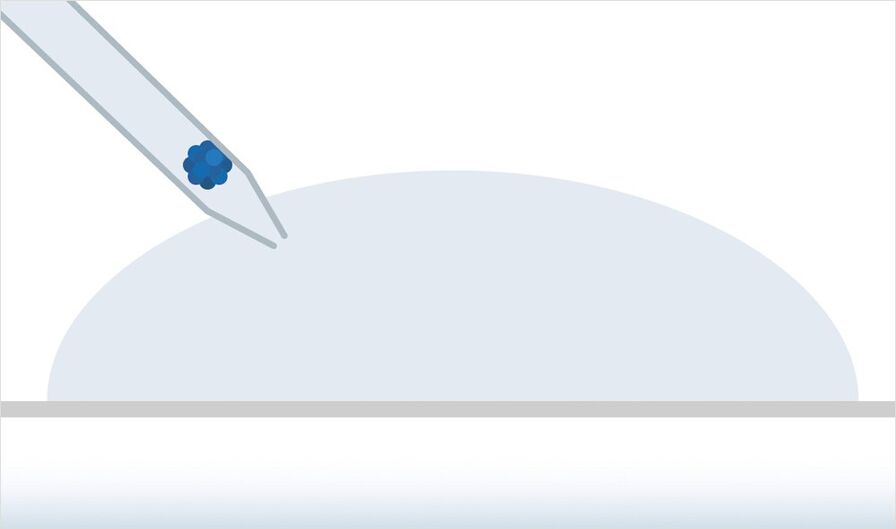
Step 1: Preparation
The sample is prepared in standard glass bottom petri dishes. No special equipment, such as capillaries, is needed. You can prepare several specimens within one experimental setup by growing several spheroids directly in a small volume of hydrogel or– alternatively by placing several drops of hydrogel inside the petri dish and transferring already developed spheroids into them.
Step 2: Mounting
The petri dish is turned upside down so that the spheroids sink into a position that guarantees optimal observability. In the next step, the hydrogel solidifies at 37°C, producing a gel that will hold the spheroids in place. Finally, the petri dish is turned upright again and filled with culturing medium.
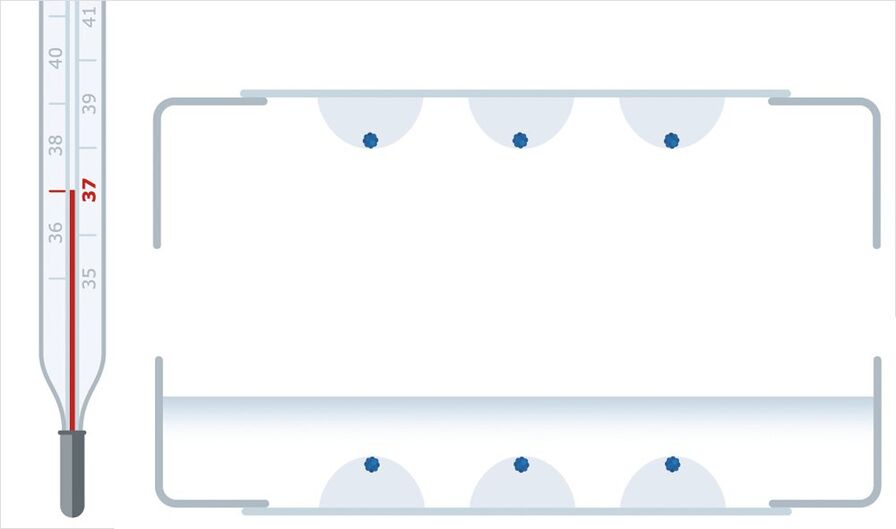
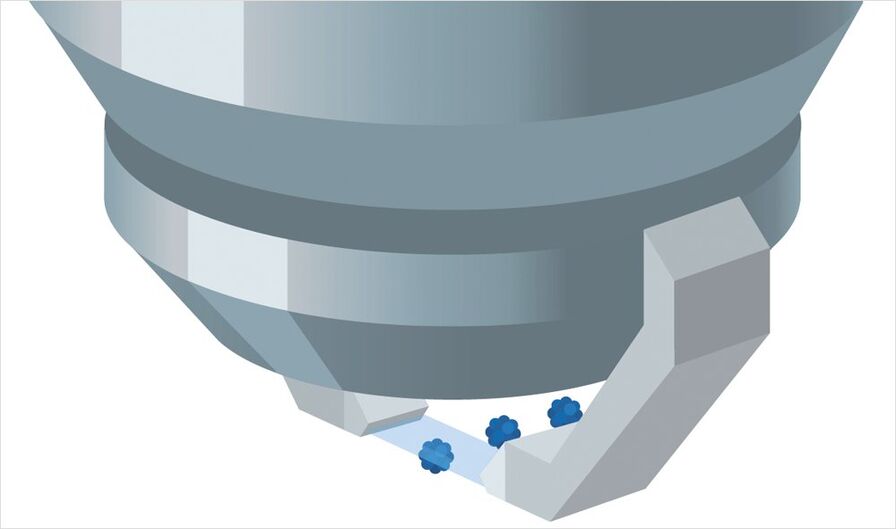
Step 3: Light Sheet Imaging
Afterwards, the petri dish is put on the sample holder of the TCS SP8 DLS . The TwinFlect mirror provides 2-sided illumination for fast and easy imaging. Thanks to the low light exposure during image acquisition, it is possible to track the natural development of spheroids over long periods of time. The design of the DLS module enables you to view several samples within a single experiment (multi-position experiment). They can be scanned at more than 60 frames per second using the full chip of an sCMOS camera, one after the other, stack by stack, and over time. For convenient culture conditions you can add incubation systems of various manufactures, e.g. Okolab and TokaiHit.
Step 4: Data Handling
Save your images as you acquire them by continuously streaming them from your temporary memory to your hard disk or a server solution (e.g. Acquifer HIVE).
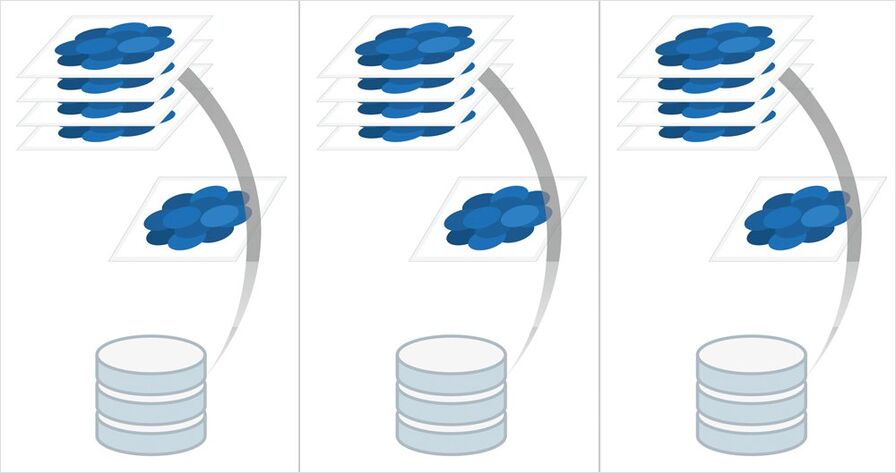

Step 5: Visualization
Once the images are acquired, you can immediately visualize your results with the 3D Visualization tool of the Leica LAS X 3D software. Look at your spheroid at a certain moment in time in 3D, or inspect a subvolume of the acquired data to visualize single cell divisions labeled with different markers.
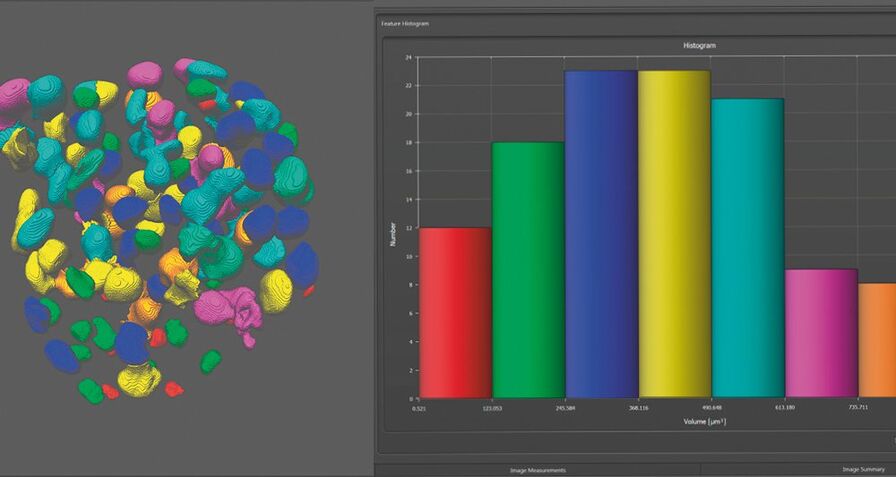
Step 6: Analysis
Comparing the behavior of spheroids that are derived from different genetic backgrounds or that have undergone different treatment is a major challenge. Data analysis is an essential tool when you need to collect quantitative information, for example on healthy and impaired tissue. A powerful 2D and 3D analysis tool is part of the Leica LAS X software.
Step 7: Share
Share your results with your colleagues, other institutions or any media - worldwide - by using generic image formats like tif or jpeg. Movies can be easily exported as mp4 videos and your quantitative results can be further evaluated in Excel reports.
Related Articles
-
Overcoming Observational Challenges in Organoid 3D Cell Culture
Learn how to overcome challenges in observing organoid growth. Read this article and discover new…
Apr 08, 2024Read article -
Notable AI-based Solutions for Phenotypic Drug Screening
Learn about notable optical microscope solutions for phenotypic drug screening using 3D-cell…
Dec 06, 2023Read article -
How to Get Deeper Insights into your Organoid and Spheroid Models
In this eBook, learn about key considerations for imaging 3D cultures, such as organoids and…
Nov 22, 2023Read article
Related Pages
-

Confocal Microscopes
Our confocal microscopes for top-class biomedical research provide imaging precision for subcellular…
Visit related page -
Neuroscience
Are you working towards a better understanding of neurodegenerative diseases or studying the…
Visit related page