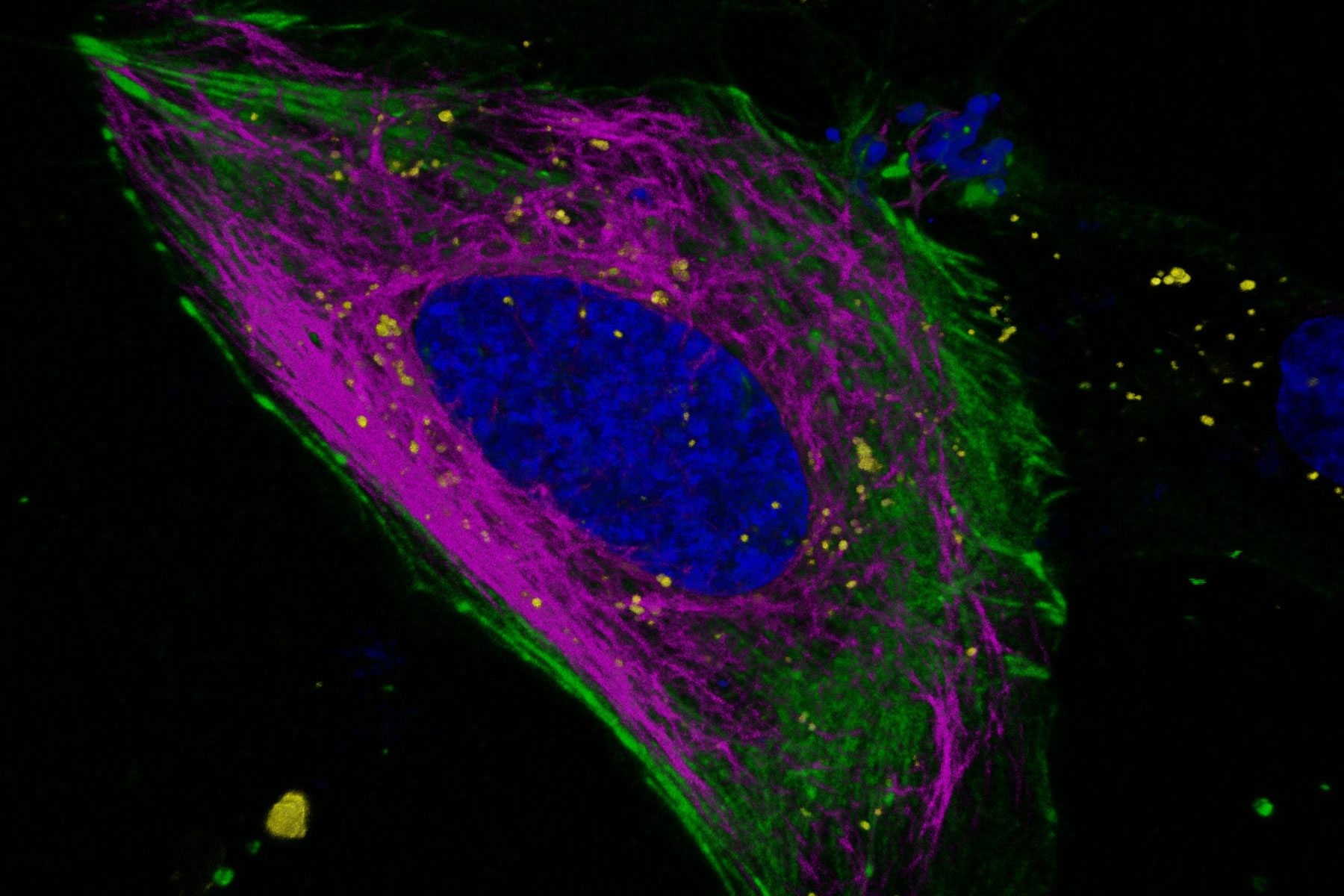
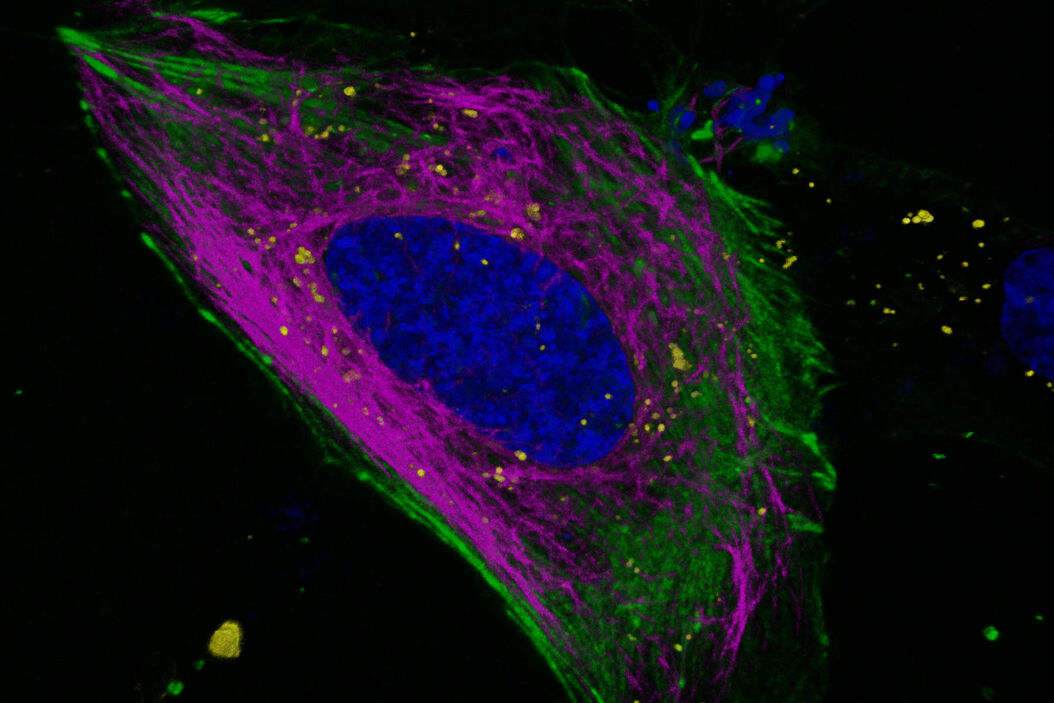
Key learnings
Read the Application Note to find out how STELLARIS can take you beyond traditional limitations:
- Image longer without photodamage, and with enhanced sensitivity for the fluorophores you use most.
- Study dynamic events at exceptionally high frame rates—capturing up to 428 frames per second without compromising spatial resolution.
- Multiplex beyond spectral options, resolving overlapping fluorescence spectra, and adding the fluorescence lifetime dimension to your experiments.
Introduction
In recent years, the field of live-cell imaging has evolved rapidly due to groundbreaking advances in confocal technology together with an ever expanding toolbox of fluorescent dyes and genetically encoded tags, as well as better biological models-such as stem cell-derived cultures, spheroids, and organoids that more closely mimic the complex physiology and architecture of in vivo systems. While great progress has been made, live cell confocal imaging still presents significant challenges when it comes to practical applications in the lab. Chief among them is how to avoid the classic trade-offs between spatial resolution, signal-to-noise ratio (SNR), and acquisition speed-all the while ensuring that cells remain healthy for the duration of the experiment.
Cell health, phototoxicity and photobleaching
Maintaining sample health is paramount to ensuring the validity of any live imaging experiment. Most tissues and cell types in multicellular organisms are shielded from light during their natural life span in vivo. Consequently, uncontrolled doses of light can perturb their behavior, activate stress responses, impair metabolic function, and alter vital structures. Phototoxicity has been reported for many popular fluorescent dyes, including Hoechst, Draq5, Fluo4-AM, Mitotracker, and Rhodamine B. When fluorophores are photobleached, the risk of phototoxicity increases, due to the generation of reactive intermediates that persist for longer, giving them more time to inflict damage [1].
Because any exposure to light has the potential to perturb living systems, there is always a limit to the length of time a live specimen can be imaged without causing damage. Great care must be taken to avoid oversampling, minimize the amount of light exposure, and control for phototoxic effects whenever possible. This fact has implications not only for experimental set-up and execution, but also when it comes to the selection of the microscopy system itself.
Fluorophore availability
Despite the growing diversity and spectral range of existing fluorophores, finding the right probes for a live-cell experiment can still be a big stumbling block. In multicolor imaging experiments, the different fluorophores in the sample need to be distinguishable from each other and bright enough to enable adequate contrast at the light dose levels needed to preserve cell health. They also need to be photostable enough to withstand repeated
imaging without photobleaching and phototoxic effects.
While fluorescent proteins (FP) are generally less photolabile than chemical dyes due to caging of the fluorophore within a protective polypeptide structure, photobleaching and phototoxicity may still be an issue depending on the experimental conditions, sample type, and FP species. Achieving sufficient brightness can be particularly problematic when working at endogenous expression levels or with weak red-emitting FPs. Spectral overlap between fluorophores can also lead to bleed-through artifacts. Endogenous fluorescence is a further complicating factor that can contribute to poor image quality and limit multiplexing capacity. Many naturally occurring fluorescent molecules-for example, NAD, FAD, lipofuscin and chlorophyll- emit in the green and yellow regions of the spectrum, where they compete with signal from commonly used fluorophores like Calcein-AM and EGFP.
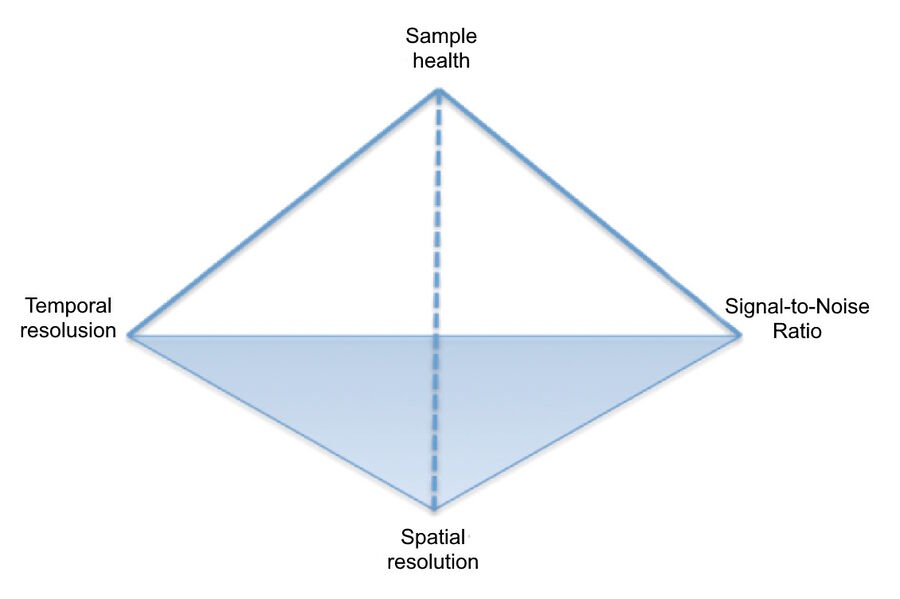
The pyramid of frustration
Three essential elements to consider when imaging live biological samples are signal-to-noise ratio (SNR), spatial resolution, and temporal resolution. Because these factors are interdependent, finding the optimal imaging conditions for a given sample usually involves compromise. For instance, to study a rapid dynamic event like calcium signaling, contrast and spatial resolution may need to be sacrificed to boost the speed of image acquisition. Such trade-offs are an everyday reality for most live cell experiments. Add to these factors the overriding priority of ensuring that the chosen settings do not damage specimen health, and users experience what has come to be known as “the pyramid of frustration” (Figure 1)-the time-consuming and extremely difficult task of balancing all four of these interdependent parameters.
Breaking the cycle of frustration: the power of STELLARIS
Addressing the challenges described above with traditional microscopy systems can be time-consuming and frustrating, with no guarantee of success. To overcome these limitations and break the “pyramid of frustration”, Leica Microsystems has designed its new STELLARIS confocal platform with the specific challenges of live-cell imaging in mind. All components have been carefully selected and harmonized to boost efficiency of photon detection, while enabling high spatiotemporal resolution with minimal sample damage. By addressing all four vertices of the pyramid in its novel design, STELLARIS makes it easier and quicker than ever to find the optimal imaging conditions for any application.
Frustration-free imaging starts with high-performance detectors
The detector is a crucial consideration for optimal imaging, since it determines the level of sensitivity to fluorescent labels in the sample, what structures can be resolved, and the degree of temporal resolution possible when capturing dynamic events. The STELLARIS Power HyD family of detectors offers users more flexibility to achieve ultra-high sensitivity across the spectrum and for varied applications. The new photon-counting capability-Power Counting-improves image contrast, for more quantitative results and a higher dynamic range [3].
The Power HyD S detectors at the core of STELLARIS provide enhanced sensitivity in the blue-green spectral range-where it matters most for many live cell experiments. With photon detection efficiencies (PDE) up to 56%, Power HyD S detectors outperform Gallium arsenide phosphide (GaAsP) PMTs, and are ideal for working with popular FP variants, including mTurquoise, CFP, GFP and YFP (Figure 2). Power HyD S detectors also provide excellent sensitivity into the red and near infrared spectral ranges.
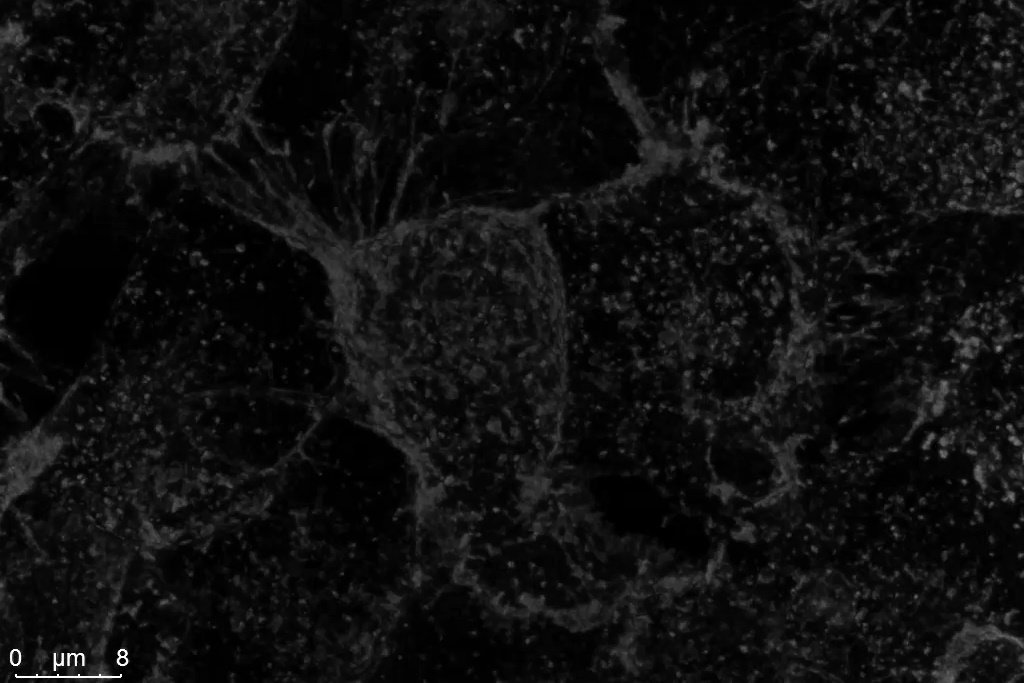
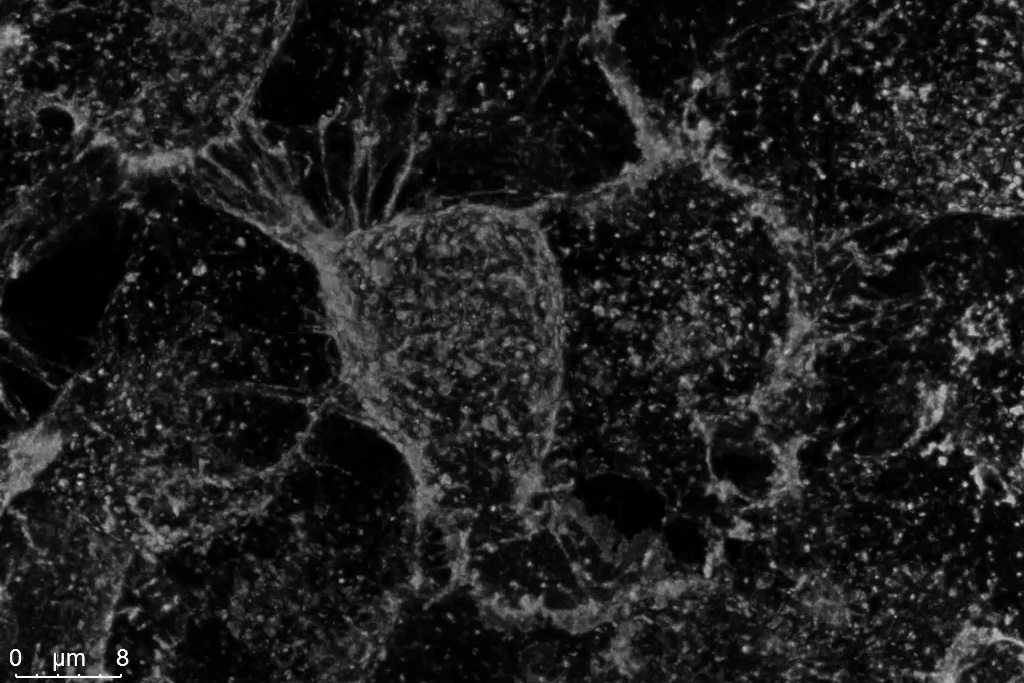
Figure 2: HeLa cells labeled with WGA-CF488. Left: Conventional photon counting; right: STELLARIS Power Counting. Shown are maximum-intensity-projections of Z-stacks acquired with a 12 kHz resonant scanner and processed by LIGHTNING.
Specialized detectors for added application versatility
Fluorescent labels that excite in the near infrared (NIR) range (720-850 nm) are especially desirable for live-cell applications because they expand multiplexing possibilities, enable greater depth of imaging, and avoid the use of wavelengths that may cause photodamage and background autofluorescence. STELLARIS Power HyD R detectors provide outstanding performance in the NIR range, which enables users to take full advantage of the additional red wavelengths accessible with the next-generation White Light Lasers (WLL) on STELLARIS 8. Together with the WLL extended excitation range on STELLARIS 8, which spans from 440nm up to 790nm, it is possible to image up to 3 additional colors in the NIR range.
Time-resolved techniques such as fluorescence lifetime imaging (FLIM) and fluorescence correlation spectroscopy imaging ( FCS ) are increasingly used to study dynamic signaling events and changes in the intracellular environment. Application of these methods in living cells requires rapid acquisition with single-photon sensitivity and high photon detection efficiency. Power HyD X are specialized detectors for time-resolved applications. Both Power HyD R and Power HyD X offer both photon counting and digital detection capabilities to extend the application flexibility of STELLARIS 8.
Avoiding trade-offs when imaging dynamic processes
When studying the dynamics of living samples, spatial resolution and contrast (SNR) are often sacrificed in order to maximize signal at high frame rates. Whether imaging very rapid events or performing reconstruction of slower processes, high frame rates can be essential to achieve sufficient resolution in both spatial and temporal dimensions. Frame-rate requirements depend on both the speed of the biological event and the spatial
resolution needed, which is related to the size of the structures of interest (Table 1).
| Process | Required frame rate (f) | Required resolution Δx' (μm) | Size of structure (μm) | Velocity v (μm/s) | References |
|---|---|---|---|---|---|
| Somite formation in chicken | 6 fph | 10 | 100 | 1.8 x 10-2 | (Palmeirim et al., 1997), (Kulesa and Fraser, 2002) |
| Mesodermal cell motion during chicken gastrulation | 1 fpm | 1 | 10 | 1.7 x 10-2 | (Zamir et al., 2006) |
| Neural crest cell migration in chicken | 2.4 fpm | 1 | 10 | 40 x 10-3 | (Kulesa and Fraser, 2000) |
| DNA broken ends in mammalian cells | 24 fpm | 2.5 x 10-3 | 25 x 10-3 | 10-3 | (Soutoglou et al., 2007) |
| Morphogen diffusion (drosophila Decapentaplegic) | 72 fpm | 2.5 x 10-3 | 25 x 10-3 | 3 x 10-3 | (Kicheva et al., 2007) |
| Ribosome translating mRNA (E. coli) | 10 fps | 4 x 10-3 | 40 x 10-3 | 4 x 10-2 | (Berg et al., 2001), (Alberts et al., 2002) |
| Flagellated cell | 100 fps | 0.1 | 1 | 10 | (Bray, 1992) |
| Calcium waves during heart development | 150 fps | 5 | 50 | 103 | (Tallini et al., 2006) |
| Kinesin I on a microtubule pulling a bead | 180 fps | 5 x 10-3 | 50 x 10-3 | 0.9 | (Nishiyama et al., 2002) |
| Embryonic zebrafish beating heart | 200 fps | 5 | 50 | 103 | (Forouhar et al., 2006) |
Table 1: Relative scale and frame rates needed for various biological processes [4].
STELLARIS combines a high-speed resonant scanner with the sensitive Power HyD detector family and rapid sampling electronics to enable exceptionally fast confocal imaging without compromising spatial resolution. A full 512x512 field of view can be captured at rates of up to 40 frames per second (fps). With the option to limit line collection in the Y direction, acquisition speed can be increased by an order of magnitude, reaching up to 428 fps. Adding to this, the Dynamic Signal Enhancement function allows users to find the optimal image quality at the required temporal resolution (Video 1).
Collect high quality images for longer without sample damage
The combination of a next-generation White Light Lasers (WLL), acousto-optical beamsplitter (AOBS), and tunable spectral detection system on STELLARIS enables optimal tuning of both excitation and detection to achieve the most efficient signal acquisition possible from each sample. The superior sensitivity of the Power HyD detector family enables the best possible image quality at the lowest needed power. With more gentle light dosages, sample integrity can be preserved for longer.
Parallel multicolor acquisition without compromising SNR
When multiplexing several fluorophores, the question of whether to perform parallel or sequential imaging is a common dilemma. Parallel acquisition can be much faster, but comes at the price of reduced SNR due to spectral bleed-through. It also significantly reduces the total time the sample is exposed to light, decreasing the chances of photo-damage. STELLARIS facilitates parallel multicolor acquisition with a WLL and AOBS for rapid tunable selection of up to 8 simultaneous excitation lines, and a matched spectral detection system comprising up to five Power HyD detectors. This capability allows simultaneous imaging of many channels without the need to move optical parts or remove crosstalk after recording.
Beyond the pyramid: unlocking the full potential of live-cell studies
In addition to overcoming the limitations and trade-offs commonly encountered with traditional imaging systems, STELLARIS integrates advanced tools and capabilities that enable researchers to extract even more information from every experiment and ask novel biological questions.
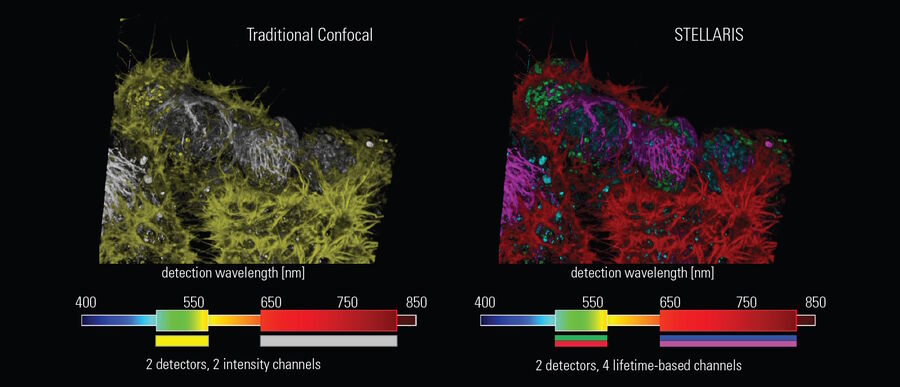
Utilize the entire spectrum
The newest WLL configurations and detector portfolio for STELLARIS 5 and 8 extend imaging capability into the NIR wavelengths, where the potential for phototoxicity is reduced. STELLARIS 8 also extends the range further into the blue spectrum, giving a range from 440 nm up to 790 nm. Both STELLARIS 5 and 8 allow defined excitation wavelengths to be chosen from anywhere within the respective excitation range, giving more flexibility and expanding experimental possibilities.
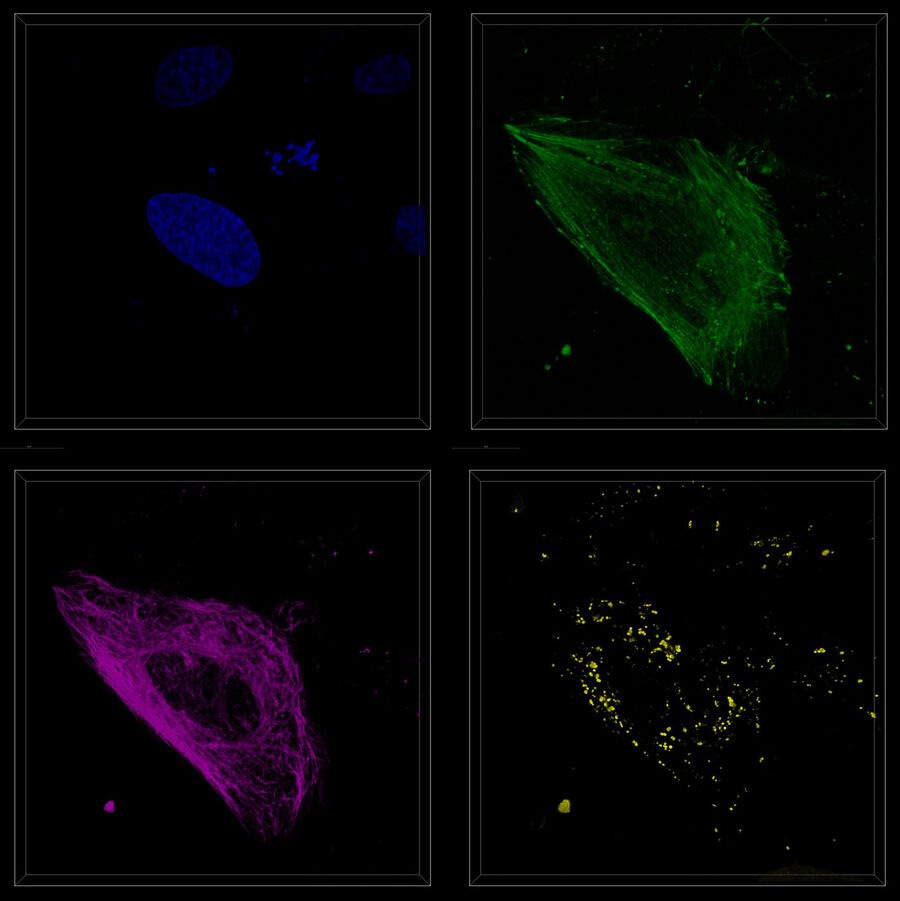
Multiplex beyond spectral options
Fluorescence lifetime is an additional characteristic of all fluorescent molecules that is orthogonal to fluorescence intensity. Fluorescence lifetime comes ‘for free’ with every experiment, and with STELLARIS it can be acquired simultaneously with the intensity measurement-without the need for additional components. TauSense is a revolutionary set of lifetime-based imaging tools that allow you to benefit from this additional information, which adds a new dimension to live cell experiments. With TauSeparation, users can separate fluorophores that have overlapping spectra based on their differing lifetime components and assign them to separate channels (Figure 4). This separation enables users to multiplex many useful live-cell probes, like mNeonGreen and Mitotracker green, based on lifetime information.
Expand experimental possibilities
With TauSense lifetime-based tools fully integrated into STELLARIS, new aspects of biology can be explored. Since many fluorophores have lifetimes that vary depending on the local microenvironment, users can take advantage of novel biosensors to monitor and quantify local variations in parameters such as pH, ion concentration and temperature. In many cases these parameters would be difficult or impossible to study by any other means.
Summary andconclusions
Performing a successful live-cell confocal imaging experiment requires careful optimization and forward planning. STELLARIS has re-imagined confocal microscopy, providing unprecedented power to maximize the amount of information gathered from each experiment, while minimizing photodamage and phototoxicity. The unique configuration of STELLARIS together with the power of TauSense lifetime-based imaging tools takes live cell imaging to a new level, expanding the range of fluorescent probes users can work with and giving them the potential to discover more.
Download
Click here to download the application note.
References
- Icha J et al. Phototoxicity in live fluorescence microscopy, and how to avoid it. Bioessays (2017) 39 (8) doi: 10.1002/bies.201700003.
- Nylk J (2017, Sep 4). Phototoxicity: The unspoken truth about live imaging. [Web log post]. Retrieved 15 Mar 2020 from https://visionformicroscopy.wordpress.com/2017/09/04/phototoxicity-the-unspoken-truth-about-live-imaging/#refs.
- Volker Schweikhard, Luis A.J. Alvarez, Irmtraud Steinmetz, M. Julia Roberti, Holger Birk, Arnold Giske. The Power HyD Family of Detectors for Confocal Microscopy-submitted for publication in Nature Methods (2020, Oct 15).
- Vermot J, Fraser SE and Liebling M. Fast fluorescence microscopy for imaging the dynamics of embryonic development. HFSP Journal (2008) 2(3):143-155.
Related Articles
-
Rapid Check of Live Stem Cells in Cell-Culture Inserts set in Multi-Well Plates
See how efficient imaging of live iPSC stem cells within cell-culture inserts set in a multi-well…
Mar 20, 2024Read article -
Notable AI-based Solutions for Phenotypic Drug Screening
Learn about notable optical microscope solutions for phenotypic drug screening using 3D-cell…
Dec 06, 2023Read article -
How Microscopy Helps the Study of Mechanoceptive and Synaptic Pathways
In this podcast, Dr Langenhan explains how microscopy helps his team to study mechanoceptive and…
Jun 22, 2023Read article
Related Pages
-

Confocal Microscopes
Our confocal microscopes for top-class biomedical research provide imaging precision for subcellular…
Visit related page -
Live Cell Imaging
Shifting perspective from single microscope components to a full working live cell imaging solution,…
Visit related page