Understanding the key players of malaria infection
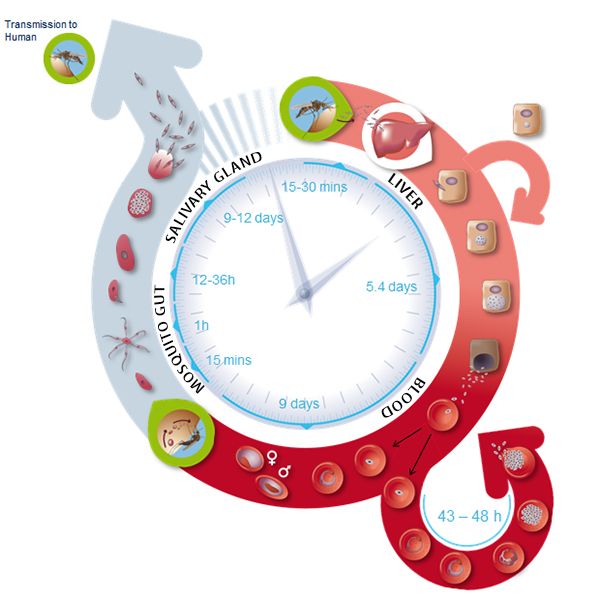
After entering the human host, the Plasmodium parasite is present in different forms during its life cycle as malaria develops (Figure 1). In the so-called blood phase [3], the merozoite form invades erythrocytes (red blood cells), evolves through different stages, and generates about 30 daughter merozoites that emerge from the infected host cell ready to attack other cells. The merozoite invasion is key to the spread of the parasite in the bloodstream and onset of the disease. Still, the molecular mechanisms of the disease remain unclear.
To invade an erythrocyte, the merozoite form must first come into contact with the host cell, the so-called “preinvasion” phase [4]. The initial interaction deforms the erythrocyte’s membrane, merozoite ligands bind to specific receptors in the membrane to create tight junctions, and a Ca2+ ion flux is triggered. The Ca2+ flux appears to be important for the cell invasion. The parasite attachment to the cell becomes irreversible and it enters the erythrocyte in less than 2 minutes.
P. falciparum reticulocyte-binding protein homologs (PfRh) and erythrocyte-binding-like proteins are important players in signaling invasion [5]. A key member of the PfRh family is PfRh5, which binds to the host protein receptor basigin. This interaction induces Ca2+ release into the erythrocyte and indicates the formation of a pore bridging the merozoite and blood cell membranes. PfRh5 is essential for merozoite invasion, as shown by studies where antibodies or soluble basigin inhibited erythrocyte infection [6]. PdRh5 works together with the PfRh5-interacting protein (PfRipr) and the cysteine-rich protective antigen (CyRPA), but the functional aspects of such interaction are unknown. Understanding the role of PfRh5/PfRipr/CyRPA is of importance as those proteins are attractive candidates for developing a vaccine that inhibits the progression of malaria at the blood phase [7].
Finding answers to malaria infection with the right tools
The Cowman group at Melbourne carried out work with genetically modified P. falciparum lines that conditionally deleted the pfripr and cyrpa genes, RiprloxCre, and CyRPAloxCre, respectively [2]. This approach was chosen over other reported strategies that were unsuccessful [6]. RiprloxCre and CyRPAloxCre parasites contain a conditional recombinase (rapamycin-induced DiCre) to regulate gene deletion fast and very efficiently, thus controlling protein levels accordingly. The authors also performed live-cell imaging and confirmed that PfRipr and CyRPA were essential for erythrocyte invasion after initial contact with the host cell. In the absence of PfRipr or CyRPA, the host cell membrane was still deformed, but the Ca2+ flux was missing, so the parasite could not enter the blood cell.
Zooming in with STED Nanoscopy
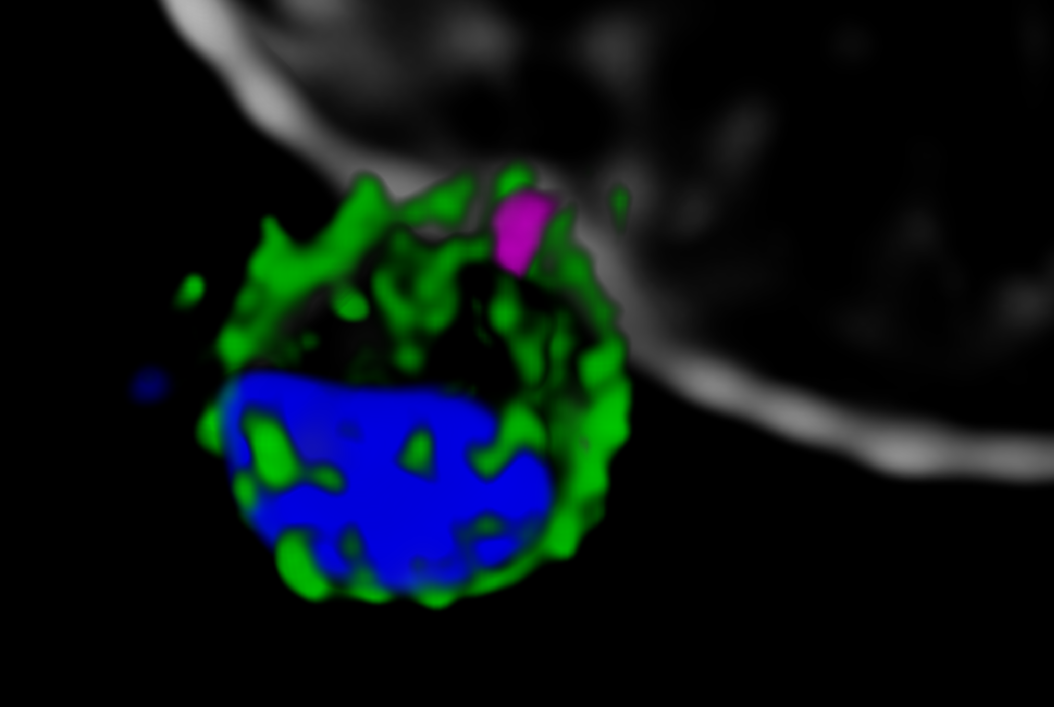
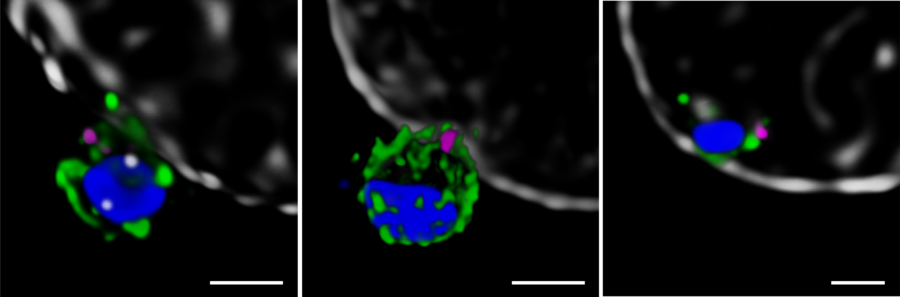
Next, the Melbourne researchers investigated the localization of PfRh5, PfRipr, and CyRPA during the invasion [2]. Confocal imaging revealed a preferential localization of the three proteins at the apical end of the parasite, the region which is preferentially in contact with the host cell. However, a precise assessment of the spatial distribution of the biomolecules is only possible with nanoscopies capable of resolving details well below the diffraction limit ¾ typically below 30 nm for 2D and 100 nm for 3D STED (stimulated emission depletion), respectively. To this end, erythrocytes were incubated with merozoites to induce invasion and labeled with antibodies against the target proteins. The samples were mounted and imaged with 2D and 3D STED nanoscopy. To further identify the initial stages of invasion, immunofluorescence labeling included a marker against RON4, a protein localizing at the apical end of the parasite (Figure 2).
The spatial information obtained with STED showed the co-localization of PfRh5, PfRipr, and CyRPA at the apical end, where RON4 was also present. The individual proteins and the bipartite PfRh5/PfRipr and CyRPA/PfRipr complexes showed a patchwork distribution over the merozoite membrane, consistent with release of the apical end. A closer, higher resolution assessment with 2D STED confirmed that PfRipr and CyRPA spread over the merozoite, but was particularly localized at the apical end. An estimation of the degree of co-localization of PfRh5/PfRipr and CyRPA/PfRipr showed a significantly larger proportion of PfRh5/PfRipr present. This finding indicates that CyRPA/PfRipr spreads onto the merozoite surface and subsequently the PfRh5/CyRPA/PfRipR complex assembles directly at the surface apical end for binding basigin. This event triggers the formation of a tight junction and enables the next steps of invasion. The presence of individual PfRh5, PfRipr, and CyRPA signals also suggests that a significant pool of each protein is still available for complex formation.
Outlook
Erythrocytic invasion is a critical phase for malaria infection in human hosts. Until recently, studies of the interaction and function of the proteins involved were limited, as suppression of the key ligand PfRh5 inhibited invasion altogether. Using conditional expression of PfRipr and CyRPA, the Cowman group revealed the essential role of PfRipr, CyRPA, and the PfRh5/PfRipr/CyRPA complex in the merozoite invasion [2]. The proteins participate in Ca2+ release into the erythrocyte through the formation and binding of the complex to the host receptor basigin. STED nanoscopy provided the necessary resolution to show that PfRh5/PfRipr/CyRPA forms at the interface between the parasite and host cell. Previous studies suggested that CyRPA linked the complex to the parasite membrane [8], but the Cowman group performed additional biochemical assays that showed this was not the case. On the contrary, the results suggest that other proteins must anchor the complex to the host cell and direct it to form at the apical end.
Future work will help find novel proteins involved in the merozoite/host cell interaction and understand the functional role of Ca2+ release in the infection pathway. Ultimately, dissecting the interaction between the host and pathogen will contribute to finding ever more effective ways to fight malaria.
References
- World Health Organization (WHO), World Malaria Report 2017 (WHO, Geneva, Switzerland, 29 November 2017)
- Volz, J.C., Yap, A., Sisquella, X., Thompson, J.K., Lim, N.T., Whitehead, L.W., Chen, L., Lampe, M., Tham, W.H., Wilson, D., Nebl, T., Marapana, D., Triglia, T., Wong, W., Rogers, K.L., Cowman, A.F. Essential Role of the PfRh5/PfRipr/CyRPA Complex during Plasmodium falciparum Invasion of Erythrocytes, Cell Host Microbe. (13 July 2016) 20 (1), 60-71, DOI: 10.1016/j.chom.2016.06.004
- Cowman, A.F., Crabb, B.S. Invasion of red blood cells by malaria parasites, Cell (2006) 124, 755–766, DOI: 10.1016/j.cell.2006.02.006
- Weiss, G.E. Gilson, P.R., Taechalertpaisarn, T., Tham, W.-H., de Jong, N.W.M., Harvey, K.L., Fowkes, F.J.I., Barlow, P.N., Rayner, J.C., Wright, G.J., Cowman, A.F., Crabb, B.S. Revealing the sequence and resulting cellular morphology of receptor-ligand interactions during Plasmodium falciparum invasion of erythrocytes. PLoS Pathog. (2015) 11 (2), e1004670, DOI: 10.1371/journal.ppat.1004670
- Tham, W.H., Healer, J., Cowman, A.F. Erythrocyte and reticulocyte binding-like proteins of Plasmodium falciparum. Trends Parasitol. (2012) 28 (1), 23–30, DOI: 10.1016/j.pt.2011.10.002
- Chen, L., Lopaticki, S., Riglar, D.T., Dekiwadia, C., Uboldi, A.D., Tham, W.-H., O'Neill, M.T., Richard, D., Baum, J., Ralph, S.A., Cowman, A.F. An EGF-like protein forms a complex with PfRh5 and is required for invasion of human erythrocytes by Plasmodium falciparum. PLoS Pathog. (2011) 7 (9), e1002199, DOI: 10.1371/journal.ppat.1002199
- Douglas, A.D., Baldeviano, G.C., Lucas C.M., Lugo-Roman L.A., Crosnier C., Bartholdson S.J., Diouf A., Miura K., Lambert L.E., Ventocilla J.A., Leiva K.P., Milne K.H., Illingworth J.J., Spencer A.J., Hjerrild K.A., Alanine D.G., Turner A.V., Moorhead J.T., Edgel K.A., Wu Y., Long C.A., Wright G.J., Lescano A.G., Draper S.J. A PfRH5-based vaccine is efficacious against heterologous strain blood-stage Plasmodium falciparum infection in Aotus monkeys, Cell Host Microbe (2015) 17 (1), 130–139, DOI: 10.1016/j.chom.2014.11.017
- Reddy, K.S. Amlabu E., Pandey A.K., Mitra P., Chauhan V.S., Gaur D. Multiprotein complex between the GPI-anchored CyRPA with PfRH5 and PfRipr is crucial for Plasmodium falciparum erythrocyte invasion, Proc. Natl. Acad. Sci. USA (2015) 112 (4), 1179–1184, DOI: 10.1073/pnas.1415466112
Related Articles
-
Extended Live-cell Imaging at Nanoscale Resolution
Extended live-cell imaging with TauSTED Xtend. Combined spatial and lifetime information allow…
Mar 05, 2024Read article -
The Guide to STED Sample Preparation
This guide is intended to help users optimize sample preparation for stimulated emission depletion…
Mar 05, 2024Read article -

Five-color FLIM-STED with One Depletion Laser
Webinar on five-color STED with a single depletion laser and fluorescence lifetime phasor…
Dec 14, 2022Read article
Related Pages
-

Confocal Microscopes
Our confocal microscopes for top-class biomedical research provide imaging precision for subcellular…
Visit related page