The laser microdissection systems Leica LMD6000 and Leica LMD7000 were used to dissect specific cells, cell populations and microscopic tissue pieces of different plants such as Dilatris species (Haemodoraceae) [1], Norway spruce (Picea abies, Pinaceae) [2, 3], Hypericum species (Hypericaceae) [4], rapeseed (Brassica napus L., Brassicaceae) [5], Colquhounia coccinea var. mollis (Lamiaceae) [6] and Cannabis sativa (Cannabaceae) [7]. The general workflow of the LMD procedure, the sample preparation for LMD (A), the laser microdissection (B), the post-excision sample treatment (C) and the analysis of the targeted plant secondary metabolites (D) is shown and discussed for each sample (Table 1). Identical or similar approaches are described in detail only once [8].
Tab. 1: Workflow of the LMD procedure.
| Step | |
|---|---|
| A | Sample preparation |
| B | Laser microdissection |
| C | Post-excision sample treatment |
| D | Measurement of plant secondary metabolites |
LMD as a tool to harvest secretory cavities from leaves of Dilatris pillansii Barker, Dilatris corymbosa Berg. and Dilatris viscosa L.
Secretory cavities from fresh Dilatris tissue
The plant families of the Haemodoraceae, Pontederiaceae, Strelitziaceae and Musaceae have in common a group of secondary metabolites known as phenylphenalenones [9]. The genus Dilatris is the only member of the Haemodoraceae family known to possess internal secretory cavities (SC), whose reddish color suggested the presence of phenylphenalenones. The use of abrasive methods to extract superficial SC from peltate glandular trichomes of some members of the Lamiaceae has been successful [10]; however the surgical dissection of internal SC accurately separating the SC from the surrounding tissue and thereby allowing the phytochemical analysis of their contents posed a new challenge [1].
(A) In the first approach, fresh leaves of D. pillansii were cut into small pieces, which were then embedded in tissue-freezing media and snap frozen in liquid nitrogen. As organic solvents are used to chemically dehydrate plant tissues in the paraffin-embedding procedure, and this can damage LMD samples and result in a complete loss of the orange colored contents of the SC, cryofixation (at –25 ºC) was deemed more appropriate to prepare tissue samples for LMD. The frozen leaf pieces were cut with a microtome and placed on a slide.
(B) The thickness of 60 µm proved to be the best to dissect the glandular epithelial cells of the SC and its secretion storage space. This was very often achieved in a single run of the laser. Leakage was observed in only a few cases and these SC were consequently discarded. The high viscosity of the phytochemical content of the SC fixed the latter to the glass slide. The 25 isolated SC were therefore picked from the glass slide under a stereomicroscope using sharp dissecting needles.
(C) The post-excision sample treatment consisted of an extraction of the secondary metabolites with an acetone:water (20:1) mixture and the removal of the cell debris using a centrifuge. The supernatant was directly transferred to a fresh microtube and dried under nitrogen.
(D) The residue was redissolved in a deuterated solvent and analyzed by reversed-phase HPLC and cryogenic 1H nuclear magnetic resonance (NMR) spectroscopy. Two major and a minor phenylphenalenone were identified comparing the 1H-NMR spectrum and HPLC chromatograms of LMD samples with those of reference compounds. The relative proportion of the detected phenylphenalenones was determined by the integral ratios of selected 1H-NMR signals. The absence of glycosidic phenylphenalenones suggested that SC are lipophilic compartments.
Secretory cavities from Dilatris herbarium tissue
We developed a new method to dissect 180-year-old SC of leaves and the outer and inner perianth segments in herbarium specimen (specifically for D. viscosa and D. corymbosa specimen). As opposed to the SC in fresh leaf material from D. pillansii, the SC of the herbarium materials were not covered by surrounding heterogeneous cells from all sides [1].
(A) A specially manufactured metal frame combined with thin glass slides allowed to fix SC located throughout the whole sample.
(B) Twenty SC were harvested using LMD.
(C) The microdissected material was collected in the lid of a microtube using gravity and then directly transferred to NMR tubes without centrifugation.
(D) Deuterated solvent was added and the mixture was sonicated for 1 min. The extracted plant metabolites were measured by cryogenic 1H-NMR spectroscopy. One phenylphenalenone was identified comparing the 1H-NMR spectrum with those of an authentic reference. The detection of a phenylphenalenone in SC of both fresh plant material and over 180-year-old herbarium specimen proved the long-term stability of this secondary plant metabolite.
LMD as a tool to explore the phytochemical composition of Norway spruce (Picea abies) bark
Stone cells
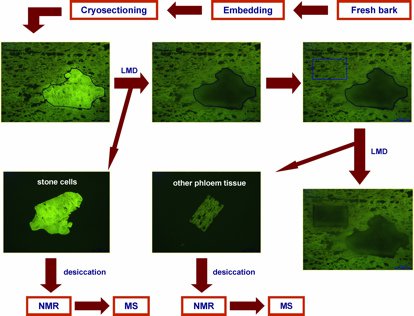
Norway spruce (Picea abies) is the most abundant conifer species in the boreal and temperate forests of Eurasia. This ecological and economic important conifer synthesizes an array of chemical defense compounds like oleoresin, a viscous mixture of mono-, sesqui- and diterpenoids, and diverse phenolics, including phenylpropanoids, stilbenes, and flavonoids as secondary plant metabolites involved in defense against pathogens and herbivores. A lot of publications have dealt with the defensive role of oleoresin while fewer have tackled the role of polyphenols which is the focus of the investigation by Schiebe et al. [11]. A combination of LMD and cryogenic NMR spectroscopy was used for the microchemical analysis of stone cells (sclereids) in the Norway spruce bark (Figure 1). Two phenolic compounds, the stilbene astringin and the dihydroflavonol dihydroxyquercetin 3'-O-β-D-glucopyranoside were identified by comparison of the NMR spectra of the extract with those of references and MS data [2].
(A) Bark pieces were sampled from trees of different heights (5 m, 15 m and 20 m) growing outdoors. A fresh razor blade was used to cut material longitudinally at a height of 1.5 m above the ground. The ice-cooled strips were transported to the laboratory and embedded in tissue freezing medium and frozen immediately with liquid nitrogen and then kept at –20 °C. The pieces were then cryosectioned at –20 °C into 30 mm slices and mounted on a standard microscope glass slide, putting them in a Leica AS LMD system with the sections facing downwards.
(B) The stone cells could be distinguished from surrounding phloem cells in fluorescence mode (excitation 450–490 nm, dicroic mirror 510 nm, emission 515 nm). The laser-microdissected material was collected in the cap of a microcentrifuge tube by gravity. Two to three days were necessary to excise each sample. The presence of excess freezing medium excluded the collection of stone cells towards the edge of the sections. After 2 h of collecting, the microtubes were removed from the LMD system to avoid excessive warming of the collected plant material by the microscope lamp.
(C) Centrifugation of the microtube allowed the material to settle on the bottom of the vessel. The desiccation of the material was performed in a TLC tank containing silica gel in the dark at 4 ºC for 48 h. The gentle conditions provided a thorough drying and minimized the residual HDO signal in the NMR spectra.
(D) The extraction was performed in a NMR tube, to which the plant material and deuterated methanol was added and which was sonicated for 5 min. Immediately thereafter, 1H-NMR, 1H-1H COSY and HSQC spectra were recorded for the determination of two phenolic compounds (astringin and dihydroxyquercetin 3'-O-β-D-glucopyranoside, as mentioned above). Rutin was used as an internal standard for quantitative analyses of the two phenolic compounds using 1H-NMR.
Fig. 1: Flow chart highlighting key steps of the combined laser microdissection (LMD)/NMR/MS protocol (modified after [2]). With kind permission from Springer Science + Business Media: Li S-H, Schneider B, and Gershenzon J: Microchemical analysis of laser-microdissected stone cells of Norway spruce by cryogenic nuclear magnetic resonance spectroscopy. Planta 225 (3): 771–79 (2007); doi: 10.1007/s00425-006-0376-z.
Phloem parenchyma cells
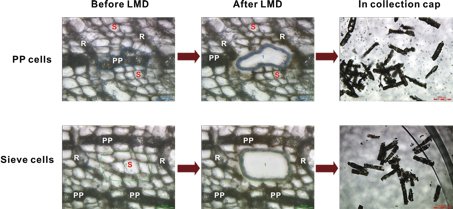
Although mature Norway spruce trees are often killed by the bark beetle Ips typographus and its associated blue-staining fungus, Ceratocystis polonica, certain genotypes of this conifer are more resistant to bark beetles or their fungal associates than others [12]. Specialized phloem parenchyma cells of the bark of Norway spruce swell and change their phytochemical content upon attack of the bark beetle and its microbial associate. LMD in combination with 1H-NMR were used to compare the phytochemical contents of normal phloem parenchyma cells and phloem parenchyma cells infected with C. polonica from the stem bark of Norway spruce (Figure 2) [3].
(A) Bark pieces from normal control and infected phloem parenchyma cells from the same tree were collected and stored at –80 ºC before use. Samples were embedded in tissue-freezing medium, snap-frozen in liquid nitrogen and cryosectioned into 40 µm thick slices.
(B) Laser microdissection was performed on a Leica LMD6000 system. In the case of C. polonica-infected samples, infected cells themselves were not collected, but cells next to the infection site were chosen for analysis. Cell layers between two healthy layers of phloem parenchyma cells were used as the source for sieve cells. The time-consuming collection required 3–5 h for each sample. The collection time of plant material for each tube was limited to 1–2 h to avoid warming up of the sample material.
(C) Invisible to the naked eye, the samples were not desiccated and directly extracted with deuterated methanol and transferred to NMR tubes.
(D) 1H-NMR spectra of the extract were immediately recorded and compared with those of phenolic references. The stilbene glucoside astringin was shown to be the major secondary metabolite located in phloem parenchyma cells and had a significantly lower concentration in adjacent sieve cells. The flavonoid (+)-catechin appeared in phloem parenchyma cells after infection by C. polonica, while the level of astringin was reduced, compared to that in non-infected cells.
Fig. 2: Laser microdissection (LMD) of polyphenolic parenchyma and sieve cells from sections of Norway spruce bark. Micrographs show sections before and after LMD (scale bars: 50 mm), and the cut in the collection cap (scale bars: 200 mm). R = ray parenchyma cells, S = sieve cells, PP = polyphenolic parenchyma cells (modified after [3]). Reprinted with permission from Li S-H et al.: Localization of phenolics in phloem parenchyma cells of Norway spruce (Picea abies). ChemBioChem 13: 2707–13 (2012). Copyright © 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
LMD as a tool to investigate metabolic profiles in dark and translucent glands of Hypericum species
Hypericum perforatum, commonly known as St. John’s wort, is one of the best-selling medicinal plants worldwide. Mild to moderate depression can be effectively treated with preparations from aerial part of this plant as proven by controlled clinical trials [13]. Flavonoids, xanthones, bioflavonoids, naphthodianthrones and especially prenylated phloroglucinols such as hyperforin and adhyperforin are the main secondary plant metabolites produced by the members of the genus Hypericum of the Hypericaceae plant family [14]. These compounds are localized in multi-cellular, globular or tunnel-shaped aggregates such as secretory canals, and dark and translucent glands. We chose a combination of modern techniques: LMD and matrix-free UV-laser desorption/ionization – mass spectrometric imaging (LDI MSI), and a classical approach: extracting whole plant material using organic solvents, to isolate and identify highly localized UV-absorbing compounds [4].
(A) To excise the dark and translucent glands from the sepals and petals of H. perforatum and H. reflexum, these were fixed between thin glass slides and a specially manufactured metal frame – similar to the procedure used to excise SC from Dilatris spp., as described above.
(B) The dissection of dark glands from petals and dark and translucent glands from leaves was performed with the nitrogen solid-state diode laser of the Leica LMD6000 system.
(C) The collected glands were briefly centrifuged and methanol was added to the microtube.
(D) After a brief sonication, aliquots of the individual sample solutions were transferred to a MALDI plate and dried on them for LDI-TOF/MS investigation. There was no matrix for ionization necessary, probably due to the strong UV absorption of the plant secondary metabolites. The LDI-TOF/MS analysis of the laser micro-dissected dark glands of the petals, the black nodules and the translucent glands of the leaves revealed the presence of different phytochemical profiles of naphthodianthrones, flavonoids and phloroglucinols in the glands of different localization.
LMD as a tool to study the defensive secondary metabolites in peltate glandular trichomes of Colquhounia coccinea var. mollis
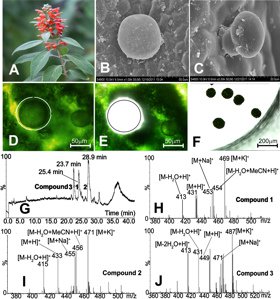
The South East Asian genus Colquhounia from the Lamiaceae plant family is a source of colquhounoids A–C, a new class of a special group of C25 terpenoids, the sesterterpenoids [15]. The leaves, buds and stems of this plant are covered by a dense layer of numerous nonglandular trichomes and peltate and capitate glandular trichomes. A combination of LMD and ultraperformance liquid chromatography/tandem mass spectrometry (UPLC/MS/MS) was used to analyze the phytochemical profile of the peltate glandular trichomes (Figure 3) [6].
(A) Freshly harvested leaves of C. coccinea var. mollis were fixed onto a specially manufactured metal frame.
(B) After mounting the slide on a Leica LMD7000 system glandular trichomes were microdissected and 500 of them were collected in one microtube.
(C) A brief centrifugation (3,000 g, 4 ºC, 3 min) settled the glandular trichomes on the bottom of the tube. For extraction, acetone was added and the vial sonicated for 10 min at 4 ºC.
(D) The supernatant was then analyzed with UPLC/MS/MS. The three major compounds: colquhounoids A–C, which had also been isolated from a methanol extract of whole leaf-material from C. coccinea var. mollis and unambiguously identified by NMR and X-ray diffraction, were found in the total ion chromatogram with retention times of 25.4, 28.9 and 23.7 min and in the positive ESI mass spectra with the molecular weights of 430, 432 and 448, respectively (Figure 4).
Fig. 4: C. coccinea var. mollis plant in bloom, (B and C) peltate and capitate glandular trichomes on the abaxial leaf surface, (D–F) collection of peltate glandular trichomes with laser microdissection, (G–J) micro-chemical analysis of secondary metabolites in the microdissected peltate glandular trichomes with UPLC/MS/MS (modified after [6]). Reprinted with permission from Org. Lett. 15: 1694–98 (2013). Copyright 2013 Americal Chemical Society.
LMD as a tool to reveal the distribution patterns of secondary metabolites in different rapeseed tissues (Brassica napus L.)
Rapeseed (Brassica napus) is grown for the production of biodiesel, animal feed and vegetable oil for human consumption. A significant part (15 %) of the global oil production is provided by this major oil crop [16]. The main storage site for lipids is the embryo of the seeds. Major secondary metabolites of the seeds of B. napus are glucosinolates and phenolic choline esters. Although these secondary metabolites have antinutritive properties [17], they are ecologically very important for the biotic and abiotic interactions of rapeseed [18]. LMD was applied to separate four tissue parts (hypocotyl and radicle, inner cotyledon, outer cotyledon and seed coat and endosperm) of seeds of B. napus from each other using LMD [5].
(A) Mature seeds of B. napus were embedded vertically in tissue-freezing medium, immediately frozen in liquid nitrogen and cryosectioned into 60 µm thick slices at –24 ºC using a cryostat microtome.
(B) The sections were directly mounted on membrane frame slides and dissected using a Leica LMD6000 laser microdissection system. The four different tissue parts were successively dissected using the strongest laser intensity and the slowest laser moving speed. The material of the four dissected groups was collected in individual microtubes by gravity including the support membrane, which was cut and harvested with the plant material.
(C) The microdissected material was then transferred to an HPLC vial and 1 mL 80 % (v/v) methanol was added to extract the secondary metabolites in an ultrasonic bath for 10 min. Additionally, sinalbin (10 µM) and cinnamic acid choline esters (10 µM) were added to the extraction solution as internal standard, sinalbin for glucosinolates and cinnamic acid choline esters for sinapine. Glucosinolates were then separated from the other compounds using anion exchange solid phase cartridges.
(D) The analysis of the non-glucosinolate compounds was performed by LC-MS. LC-DAD/MS was used to analyze the glucosinolates in their desulphated form by comparing MS data and retention times with those of references. The results indicated the even distribution of glucosinolates in mature rapeseed embryo tissues. The even distribution of sinapine as the dominant phenolic compound in rapeseed embryo tissues supports its depot function. Additionally compounds of the non-glucosinolate fraction such as cyclic spermidine conjugates occurred only in the hypocotyl and radicle and two flavonoids found mostly in cotyledons.
LMD as a tool to excise trichomes of Cannabis sativa for cannabinoid analysis
Cannabis sativa is an annual dioecious plant. Originating from Eastern and Central Asia it has long been cultivated as a source for traditional medicine [19]. The compounds responsible for the biological activities of C. sativa seem to be phytocannabinoids, a unique group of terpenophenolics possessing alkylresorcinol and monoterpene moieties. The main sites of cannabinoid and essential oil production are trichomes, especially the capitate-stalked glandular hairs. The secondary metabolites are produced mostly during the last five weeks of the total flowering cycle of eight weeks. Therefore glandular trichomes from week 4 to 8 of the flowering period were harvested by LMD and the cannabinoid profile analyzed by LCMS and by cryogenic NMR.
(A) Bracts and flowers were cut into small slices (length 0.2–0.5 cm) and the pieces fixed in 4 % phosphate-buffered formaldehyde, exposed to vacuum for 10 min, infiltrated overnight at –4 °C and then mounted on slides with steel frames (PET-membrane, 25 mm × 76 mm) [20].
(B) Intact capitate-stalked trichomes, heads and stems, as well as intact capitate sessile hairs were dissected using a Leica LMD6000 microscope. From each sample, 25–143 trichomes were excised and collected by gravity in Eppendorf tubes. The microdissected material was then vortexed for 1 min and centrifuged for 5 min in a microfuge. To avoid decarboxylation and degradation of cannabinoid, the dissected samples were stored at –20 °C directly after LMD [21].
(C) For LC-MS analysis, 60 µL methanol was added to each microdissected sample from week 4, 5, 6, 7, and 8, sonicated for 10 min and incubated overnight at room temperature. The samples were then diluted in a H2O/MeOH (2/1, v/v) + 0.1 % formic acid solution. For cryogenic NMR measurement, a minimum of deuterated chloroform was added to dissected capitate-stalked and capitate-sessile trichomes harvested at week 8. After sonification for 1 min, the solution was transferred to NMR tubes for analysis.
(D) LC-MS and NMR data identified Δ9-tetrahydrocannabinolic acid (THCA) and cannabidiolic acid (CBDA) as the predominant compounds of the capitate-stalked and capitate-sessile trichomes. LC-MS analysis data were used for a comparative study of cannabinoids profiles of microdissected trichomes collected over the last 5 weeks of the growth cycle. The results showed qualitative similarity of the investigated samples. THCA, CBDA, cannabigerolic acid (CBGA), Δ9-tetrahydrocannabinol, cannabidiol and cannbigerol were identified in all samples during flowering stages. Cannabichromene and cannabinol were detected as minor compounds only in the samples of intact capitate-stalked trichomes and their heads harvested at week 8.
Conclusion
The present overview of our LMD-based publications shows the broad diversity of plant material which can be prepared to get localized phytochemical information on a cellular level (leaf, stem, flower and seed material of different species from different plant families). LMD-based chemical analysis can reveal the uneven and plant-developmental-stage-dependent accumulation of secondary metabolites in special tissues. Therefore the procedure can be used to determine different, organ-dependent biological functions of secondary metabolites. Furthermore information about the specific localization of metabolites may be useful to study biosynthetic pathways, the modification of metabolites and the regulation of their concentration. Different plant material preparation methods for LMD such as the direct use of sample material in specially manufactured slide systems or the embedding of sample material before the cryosectioning of the material were presented. We are nevertheless aware of the challenge to develop modified or new analytical strategies for the preparation of each new sample. Further improvements in the field of analytical methods can minimize the amount of material necessary for successful identification. Therefore the work load to analyze such tissues and compounds can be reduced allowing the study of these difficult to isolate sample materials.
Acknowledgements
We thank Alexandra zum Felde for editorial help. The authors gratefully acknowledge Daniel Veit for constructing special slides for LMD.
References
- Hölscher D, and Schneider B: Laser microdissection and cryogenic nuclear magnetic resonance spectroscopy: an alliance for cell type-specific metabolite profiling. Planta 225: 763–70 (2007).
- Li S-H, Schneider B, and Gershenzon J: Microchemical analysis of laser-microdissected stone cells of Norway spruce by cryogenic nuclear magnetic resonance spectroscopy. Planta 225: 771–79 (2007).
- Li S-H, Nagy NE, Hammerbacher A, Krokene P, Niu X-M, Gershenzon J, and Schneider B: Localization of phenolics in phloem parenchyma cells of Norway spruce (Picea abies). ChemBioChem 13: 2707–13 (2012).
- Hölscher D, Shroff R, Knop K, Gottschaldt M, Crecelius A, Schneider B, Heckel DG, Schubert US, and Svatoš A: Matrix-free UV-laser desorption/ionization (LDI) mass spectrometric imaging at the single-cell level: distribution of secondary metabolites of Arabidopsis thaliana and Hypericum species. Plant Journal 60: 907–18 (2009).
- Fang JJ, Reichelt M, Hidalgo W, Agnolet S, and Schneider B: Tissue specific distribution of secondary metabolites in rapeseed (Brassica napus L.) PLoS One 7: e48006 (2012).
- Li C-H, Jing S-X, Luo S-H, Shi W, Hua J, Liu Y, Li X-N, Schneider B, Gershenzon J, and Li S-H: Peltate glandular trichomes of Colquhounia coccinea var. mollis harbor a new class of defensive sesterterpenoids. Organic Letters 15: 1694–97 (2013).
- Happyana N, Agnolet S, Muntendam R, van Dam A, Schneider B, and Kayser O: Analysis of cannabinoids in laser-microdissected trichomes of medicinal Cannabis sativa using LCMS and cryogenic NMR. Phytochemistry 87: 51–59 (2013).
- Fang JJ, and Schneider B: Laser microdissection: a sample preparation technique for plant micrometabolic profiling. Phytochemical Analysis (2013); doi: 10.1002/pca.2477.
- Cooke R, and Edwards JM: Naturally occurring phenalenone and related compounds. Progress in the Chemistry of Organic Natural Products 40: 153–90 (1980).
- Gershenzon J, McCaskill D, Rajaonarivony JIM, Mihaliak C, Karp F, and Croteau R: Isolation of secretory cells from plant glandular trichomes and their use in biosynthetic studies of monoterpenes and other gland products. Analytical Biochemistry 200: 130–38 (1992).
- Schiebe C, Hammerbacher A, Birgersson G, Witzell J, Bordelius PE, Gershenzon J, Hansson BS, Krokene P, and Schlyer F: Inducibility of chemical defenses in Norway spruce bark is correlated with unsuccessful mass attacks by the spruce bark beetle. Oecologia 170: 183–98 (2012).
- Krokene P, Solheim H, Krekling T, and Christiansen E: Inducible anatomical defense responses in Norway spruce stems and their possible role in induced resistance. Tree Physiology 23: 191–97 (2003).
- Müller WE: Current St. John’s wort research from mode of action to clinical efficacy. Pharmacological Research 101–109 (2003).
- Wolfender J-L, Verotta L, Belvisi L, Fuzzati N, and Hostettmann K: Structural investigations of isomeric oxidized forms of hyperforin by HPLC-NMR and HPLC-MS. Phytochemical Analysis 14: 290–97 (2003).
- Hog, DT, Webster R, and Trauner D: Synthetic approaches toward sesterterpenoids. Natural Product Reports 29: 752–79 (2012).
- Wolfram K, Schmidt J, Wray V, Milkowski C, Schliemann W, and Strack D: Profiling of phenylpropanoids in transgenic low-sinapine oilseed rape (Brassica napus). Phytochemistry 71: 1076–84 (2010).
- Nesi N, Delourme R, Brégone M, Falentin C, and Renard M: Genetic and molecular approaches to improve nutritional value of Brassica napus L. seed. Comptes Rendus Biologies 331: 763–71 (2008).
- Wink M: Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 64: 3–19 (2003).
- Zuardi AW: History of cannabis as a medicine: a review. Revista Brasileira de Psiquiatria 28: 153–57 (2006).
- Olsson ME, Olofsson LM, Lindahl A-L, Lundgren A, Brodelius M, and Brodelius PE: Localization of enzymes of artemisinin biosynthesis to the apical cells of glandular secretory trichomes of Artemisia annua L. Phytochemistry
70 (9): 1123–28 (2009). - Lewis GS, and Turner CE: Constituents of Cannabis sativa L. 13. Stability of dosage from prepared by impregnating synthetic (–)-Δ9-trans-tetrahydrocannabinol on placebo Cannabis plant material. Journal of Pharmaceutical Sciences 67: 876–78 (1978).
Related Articles
-
Exploring Subcellular Spatial Phenotypes with SPARCS
Discover spatially resolved CRISPR screening (SPARCS), a platform for microscopy-based genetic…
Sep 26, 2023Read article -

How is Microscopy Used in Spatial Biology? A Microscopy Guide
Different spatial biology methods in microscopy, such as multiplex imaging, are helping to better…
May 10, 2023Read article -
RNA Quality after Different Tissue Sample Preparation
The influence of sample preparation and ultraviolet (UV) laser microdissection (UV LMD) on the…
Sep 23, 2022Read article