Introduction
The mechanisms behind DED are not well understood. DED usually presents with mild discomfort. However, blurred vision and a reduction in quality of life can occur with prolonged disease [1]. While DED is quite common, its pathogenesis still remains unclear. In particular, the role of mucin proteins and their level of glycosylation in the development and progression of DED is not well known [1]. In the eye, transmembrane and soluble secreted mucins synergistically provide barrier and lubrication functions against environmental interrogations. The focus of this study is to visualize the presence of O-glycans on cell membranes which is characteristic of mammalian glycoproteins, such as mucin 1. Surprisingly, a high concentration of O-glycans was also observed on membrane ridges, supporting their potential role in epithelial barrier functions. In addition, mucin 1 is hypothesized to serve as a boundary lubricant, assisting blink motions. A dysfunction in either lubrication or barrier processes can potentially lead to DED. Here, human corneal epithelial cells were used to assess the level of glycosylated mucin 1 expression.
Challenges
When imaging mucins on the surface of stratified human corneal cells, confocal microscopy can be used to take z-stacks of the protein monolayers, but it can end up being quite time-consuming. A solution that can quickly image the mucins on the cell surfaces and achieve sharp, high-contrast 3D imaging, where important details are clearly resolved, is more practical. Conventional widefield microscopy is fast and offers detection sensitivity, but unfortunately images of thick specimens often show an out-of-focus blur or haze which reduces the contrast [2].
Methods
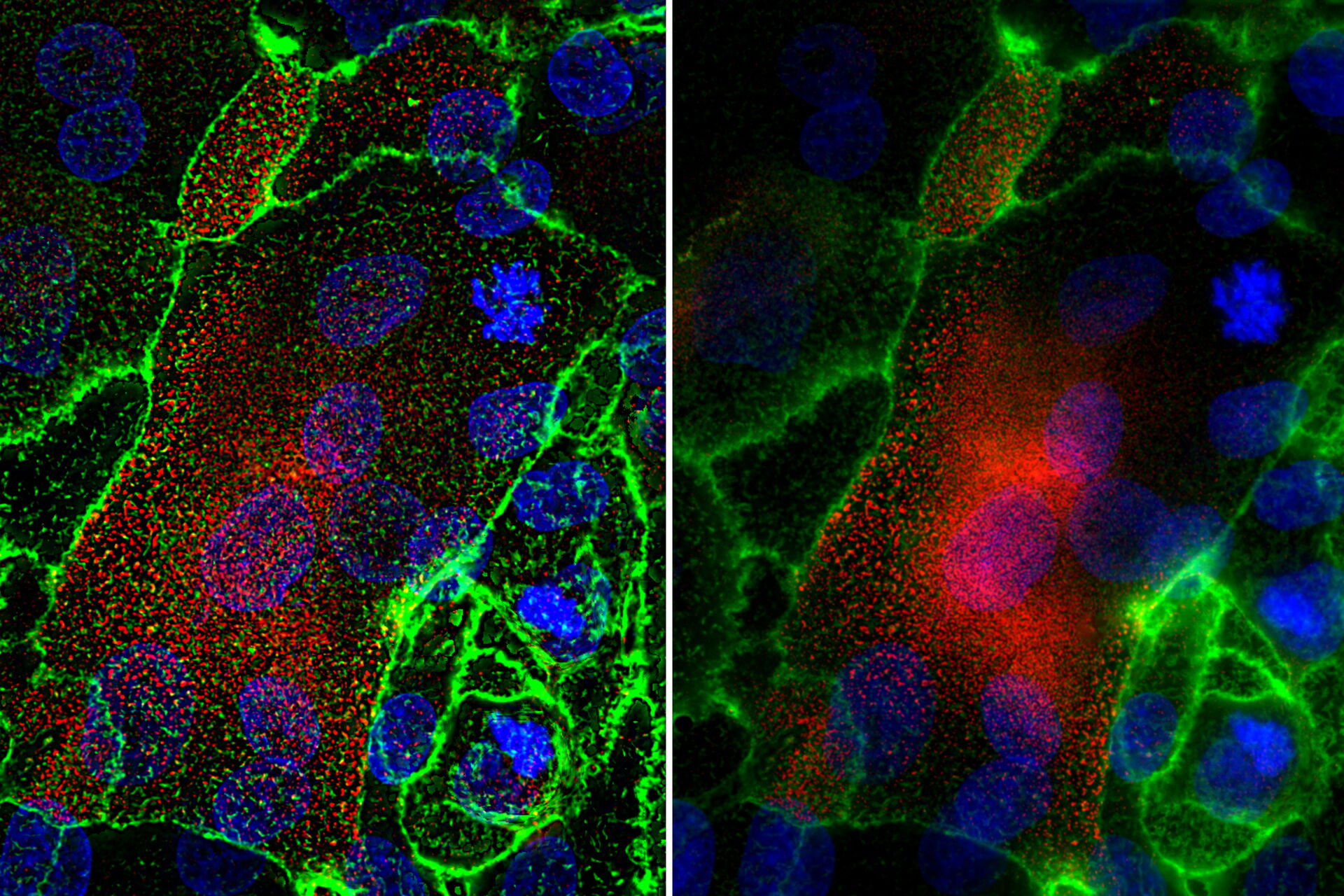
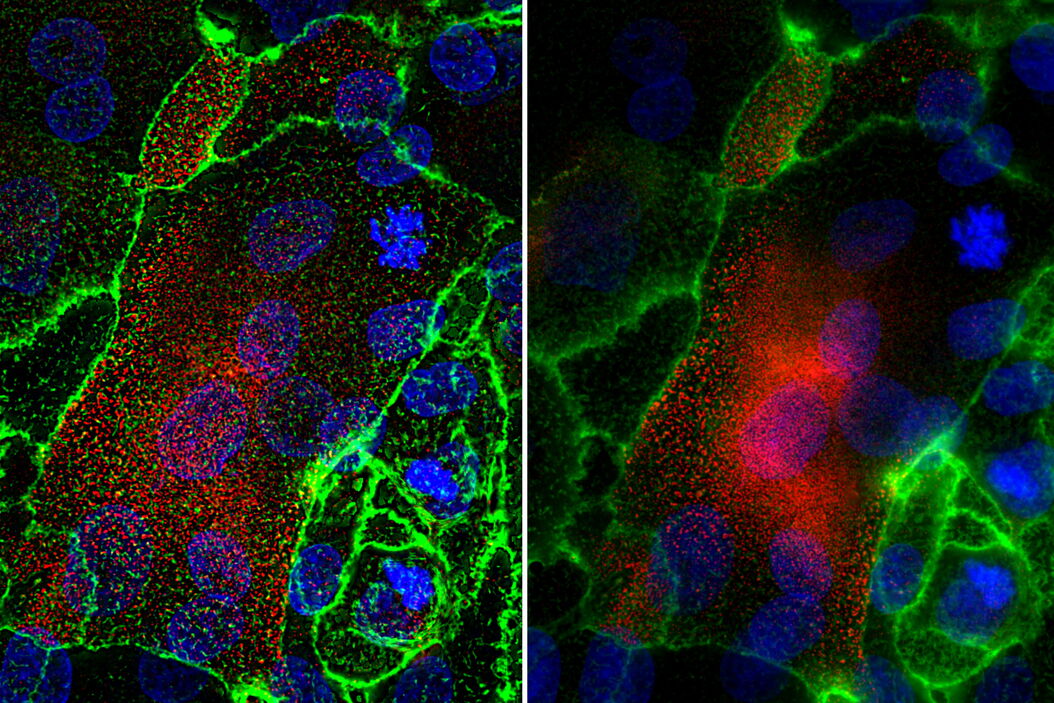
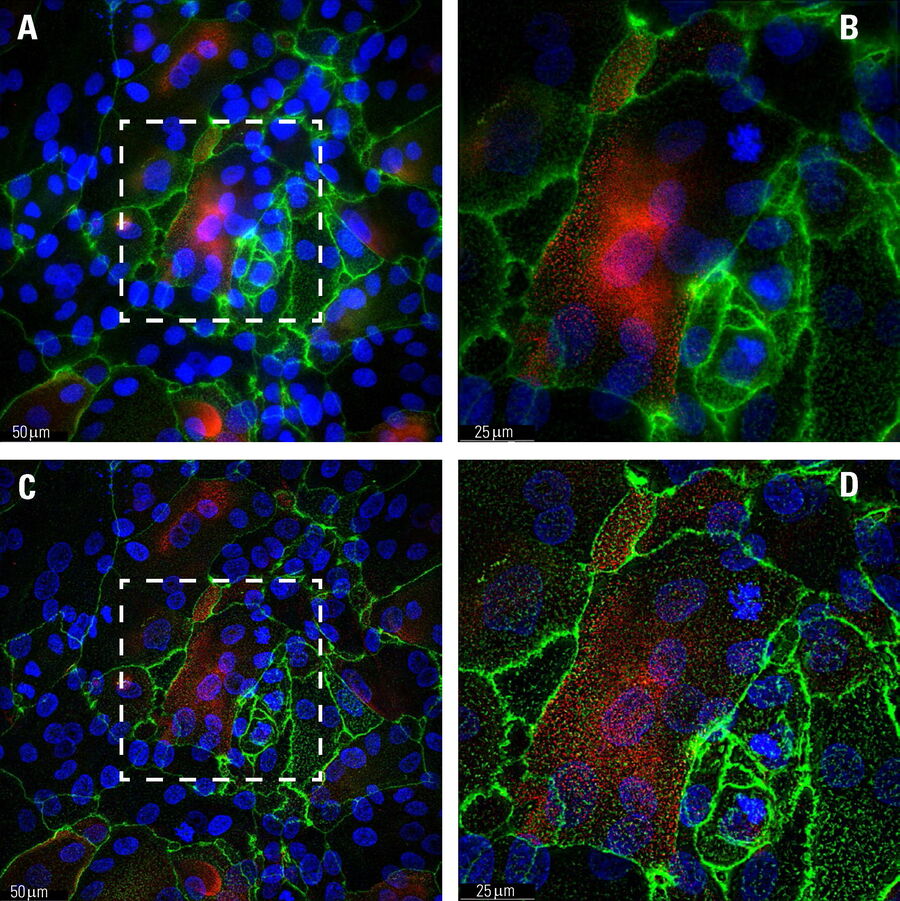
Stratified human corneal epithelial cells were fixed and immunofluorescently stained on day 3 with Hoechst (blue, marking DNA in the nucleus), Alexa fluorophore conjugated anti-bodies for mucin1 (red), and fluorescein-labeled jacalin lectin (green) [1]. Jacalin staining indicates the presence of O-glycans on cell membranes. The cells were imaged with a THUNDER Imager Tissue. A z-stack comprised of 44 slices representing a total thickness of 11.8 µm was taken using 3 channels in under 40 seconds (Figure 1). LVCC (Large Volume Computational Clearing) was applied to the image followed by an Extended Depth of Field (EDoF) projection.
Results
The use of THUNDER technology to optodigitally remove the out-of-focus blur resulted in the ability to resolve membrane ridges not seen when imaging with conventional widefield microscopy. The combination of LVCC and EDoF allows the details from all planes in one projection image to be clearly made out.
Conclusions
The THUNDER technology Large Volume Computational Clearing (LVCC) [2] significantly enhances the contrast when imaging human corneal epithelial cells, allowing the membrane ridges to be resolved which was not achieved with conventional widefield imaging.
References
- C. Liu, A.C. Madl, D. Cirera-Salinas, W. Kress, F. Straube, D. Myung, G.G. Fuller, Mucin-Like Glycoproteins Modulate Interfacial Properties of a Mimetic Ocular Epithelial Surface, Advanced Science (2021) 2100841, DOI: 10.1002/advs.202100841.
- J. Schumacher, L. Bertrand, THUNDER Technology Note: THUNDER Imagers: How Do They Really Work? Science Lab (2019) Leica Microsystems.
Related Articles
-
What are the Challenges in Neuroscience Microscopy?
eBook outlining the visualization of the nervous system using different types of microscopy…
Jun 14, 2023Read article -
Central Nervous System (CNS) Development and Activity in Organisms
This article shows how studying central nervous system (CNS) development in Drosophila-melanogaster…
May 12, 2023Read article -
Going Beyond Deconvolution
Widefield fluorescence microscopy is often used to visualize structures in life science specimens…
Mar 22, 2023Read article