Neural crest cells
The neural crest (NC) is an embryonic cell population with remarkable multipotency and migratory ability enabling it to contribute to the development of diverse organ systems, including the craniofacial skeleton. i.e., the bone and cartilage of the face and skull, and the peripheral nervous system [1,2]. The dysregulation of cranial NC development often results in unusual craniofacial development, so studying the NC can help lead to a better understanding of how neurocristopathies can be prevented [1,2]. Researchers in developmental biology and embryology are interested in understanding the mechanisms underlying the specification, migration, and differentiation of NC cells in multiple vertebrate embryos. It is also known that NC cells normally downregulate levels of the cadherin-6B cell adhesion molecule (CAM).
Challenges when imaging embryos
Thick embryo specimens for developmental biology studies can be challenging for widefield microscopy, because there is often an out-of-focus blur or “haze” due to light scattering present in the images [3]. By removing the haze, then structures deep inside the specimen are better revealed.
Methods
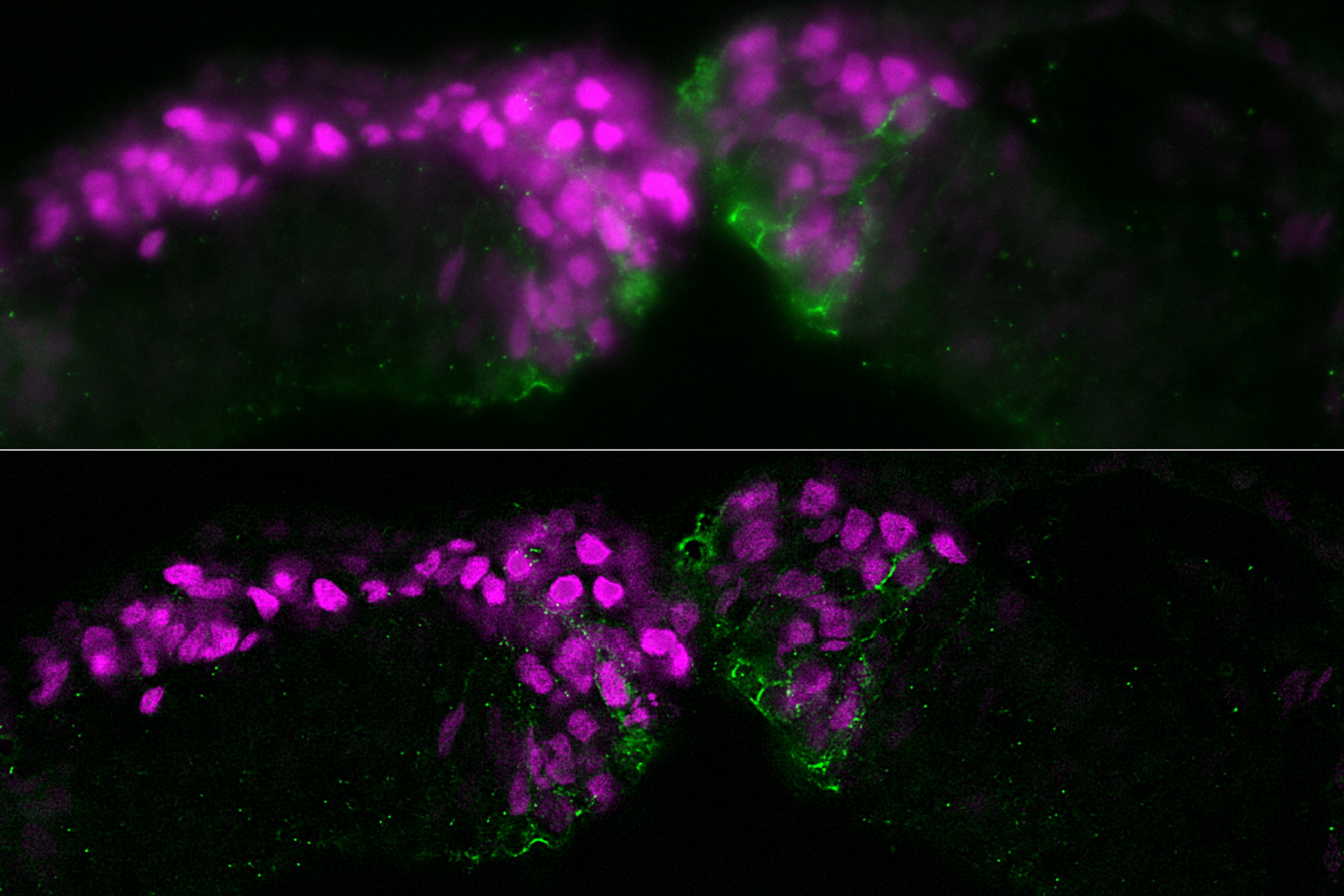
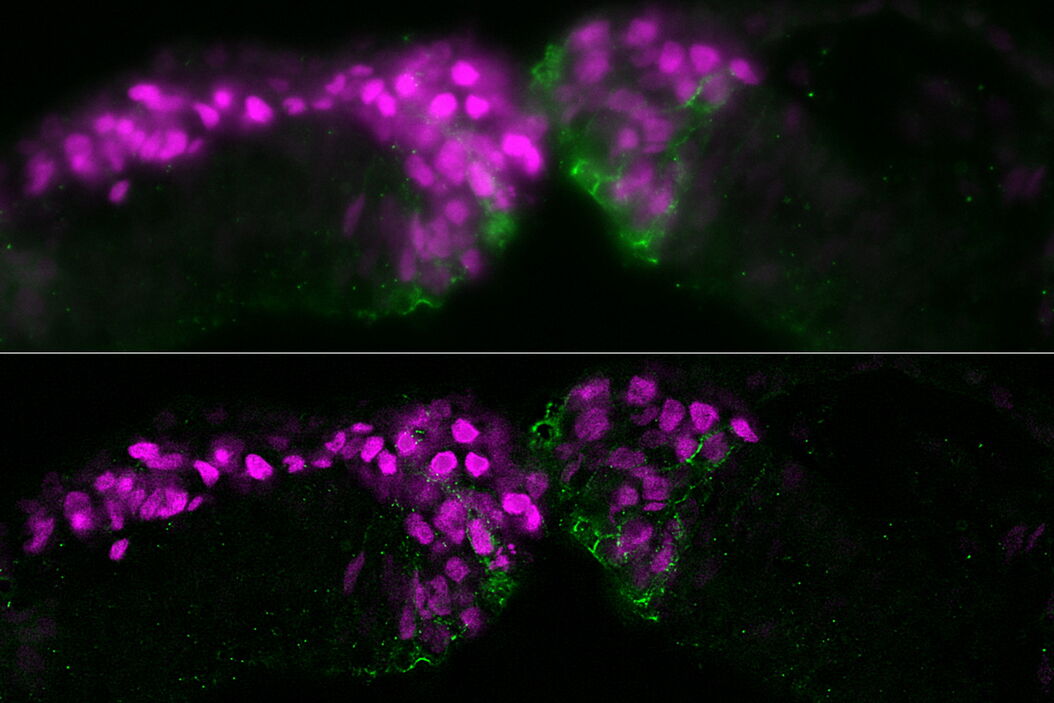
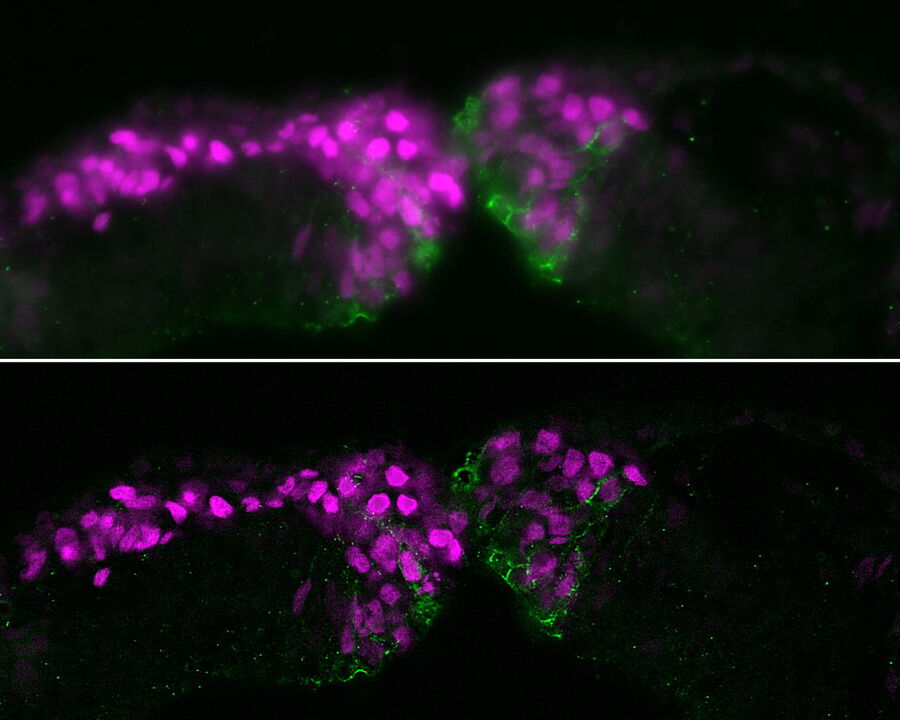
The specimen studied was a cross section of a chicken embryo at the level of the midbrain showing neural crest (NC) cells. NC cells appear as magenta and Cadherin-6B adhesion molecules appear as green. Fluorescence imaging was done with a THUNDER Imager 3D Assay using a 40x, 1.3 numerical aperture (NA), plan apochromat, oil-immersion objective. Small volume computational clearing (SVCC) was applied [3,4].
Results imaging neural crest cells
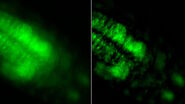
The images below are a raw widefield image and THUNDER image of a chicken embryo. The NC cells (magenta) undergo epithelial-to-mesenchymal transition and begin to migrate. Cadherin-6B (green) begins to migrate laterally to the left side of image. NC cells in which the epithelial-to-mesenchymal transition is experimentally blocked are on the right side of image. Cadherin-6B levels remain high and the NC fails to leave the neural tube epithelium.
Conclusions
The image results shown demonstrate that a THUNDER Imager 3D Assay is able to visualize more clearly the neural crest cells and fluorescence signals of cadherin molecules in the embryo specimen compared to a conventional widefield microscope system.
References
- M.L. Piacentino, E.J. Hutchins, M.E. Bronner, Essential function and targets of BMP signaling during midbrain neural crest delamination, Developmental Biology (2021) vol. 477, pp. 251-261, DOI: 10.1016/j.ydbio.2021.06.003.
- M.L. Piacentino, Y. Li, M.E. Bronner, Epithelial-to-mesenchymal transition and different migration strategies as viewed from the neural crest, Current Opinion in Cell Biology (2020) vol. 66, pp. 43-50, DOI: 10.1016/j.ceb.2020.05.001.
- J. Schumacher, L. Bertrand, Real time images of 3D specimens with sharp contrast free of haze: Technology Note THUNDER Imagers: How Do They Really Work? Science Lab (2019) Leica Microsystems.
- L. Felts, V. Kohli, J.M. Marr, J. Schumacher, O. Schlicker, An Introduction to Computational Clearing: A New Method to Remove Out-of-Focus Blur, Science Lab (2020) Leica Microsystems.
Related Articles
-
What are the Challenges in Neuroscience Microscopy?
eBook outlining the visualization of the nervous system using different types of microscopy…
Jun 14, 2023Read article -
Central Nervous System (CNS) Development and Activity in Organisms
This article shows how studying central nervous system (CNS) development in Drosophila-melanogaster…
May 12, 2023Read article -
Going Beyond Deconvolution
Widefield fluorescence microscopy is often used to visualize structures in life science specimens…
Mar 22, 2023Read article