Introduction
Vascular diseases of the retina are a major cause of impaired vision and blindness [1]. Retinas in the majority of mammals are perfused by three layers of vascular networks that include two intraretinal capillary beds. In humans, genetic mutations cause familial exudative vitreoretinopathy (FEVR), an inherited disease characterized by incomplete vascularization of the peripheral retina, Norrie disease, retinopathy of prematurity (ROP), or Coat’s disease [1]. The retinal vasculature is altered in each of these diseases. Scientists use the mouse retina model to study interactions between endothelial cells, blood vessels, microglia, and astrocytes which happen in the retina. The whole-mount retina preparation is exploited to visualize its vasculature [1]. Following fixation and staining, the whole retina must be imaged at high resolution to provide an overview of the whole vascular network, as well as single cell interactions. The results reported here demonstrate how interactions between cells in mouse retina can be studied efficiently with a THUNDER Imager 3D Cell Culture and Large Volume Computational Clearing (LVCC) [2,3].
Challenges
To image whole retina in a practical way, it is helpful to have a solution that can quickly achieve sharp, high-contrast 3D images where important details are clearly resolved. The retina imaging is typically done using tile scanning with a confocal microscope, but this approach takes several hours to acquire an entire image. Conventional widefield microscopy is fast and offers detection sensitivity, but unfortunately images of thick specimens, like whole retina, often show an out-of-focus blur or haze which reduces the contrast [2,3].
Methods
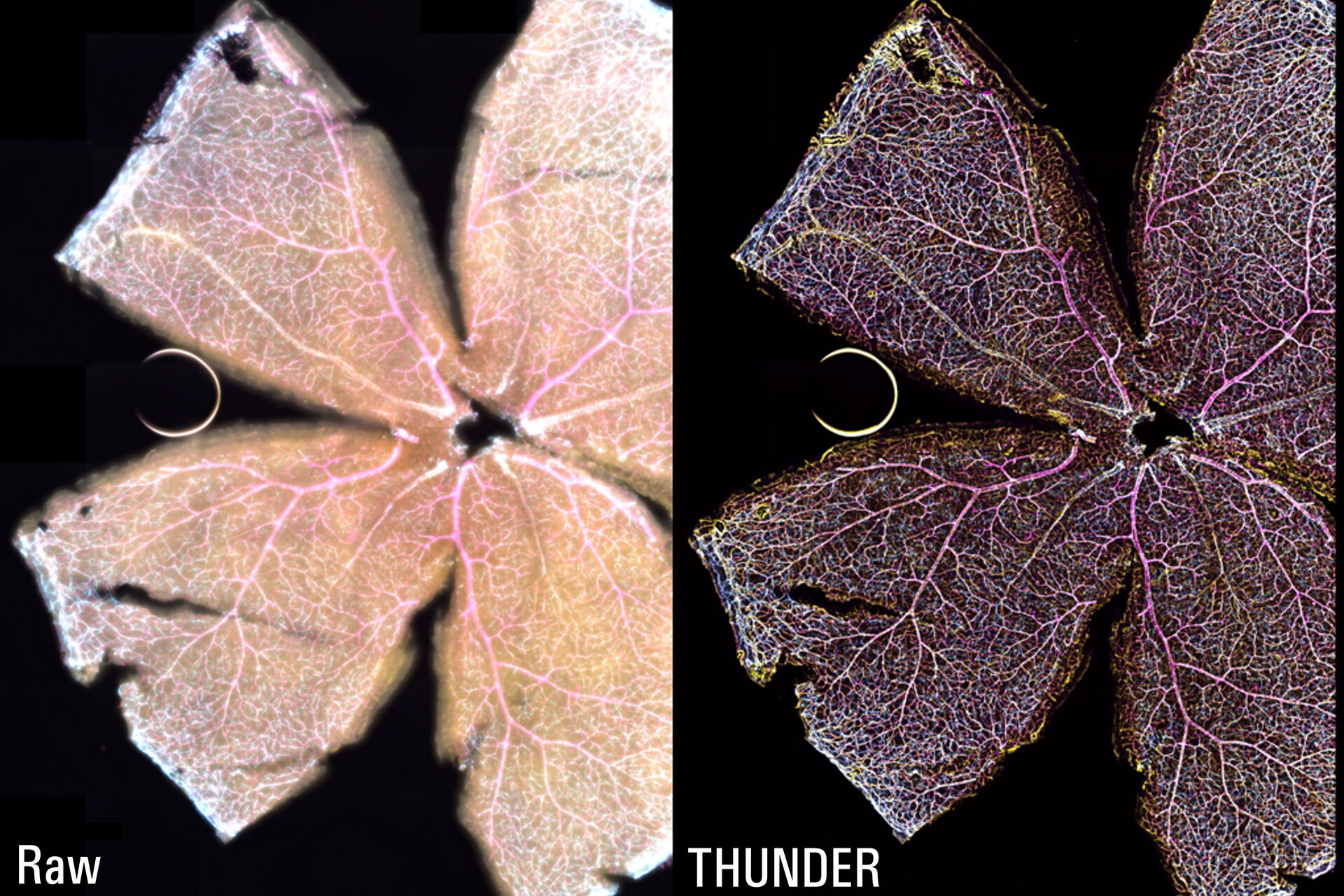
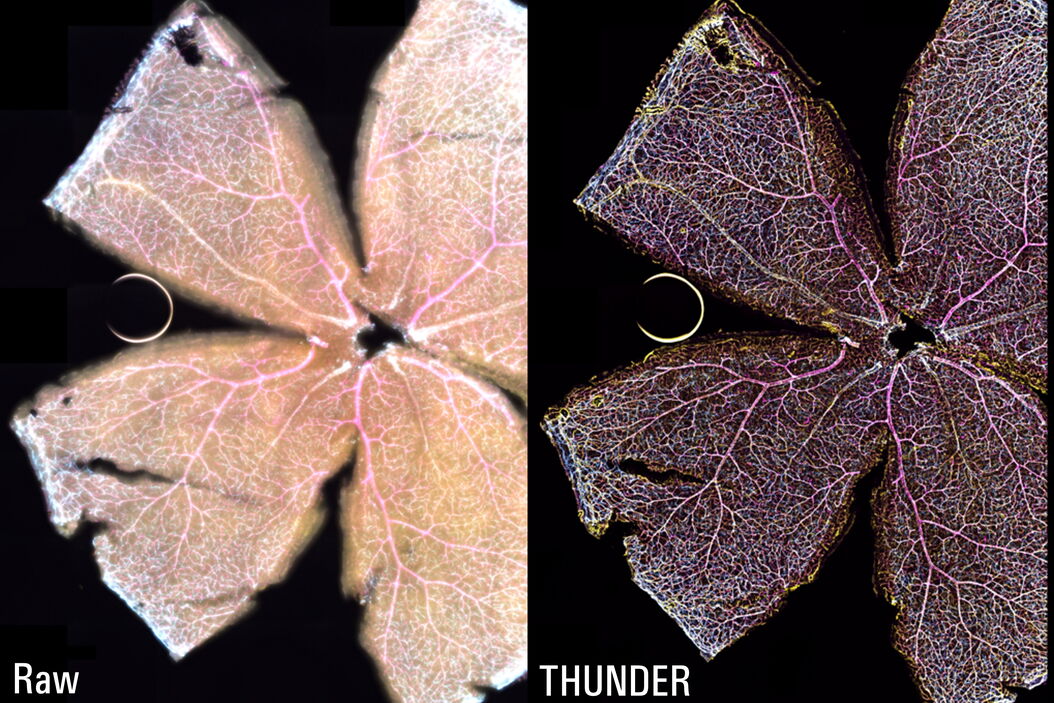
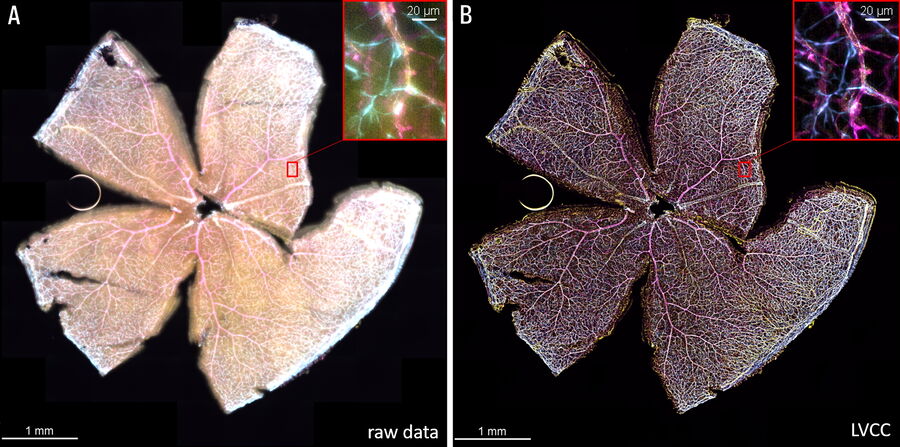
The whole-mount retina preparations were imaged with a THUNDER Imager 3D Cell Culture. The retina were labelled with anti-CD31 antibodies to indicate endothelial cells (yellow), IsoB4 for blood vessels and microglia (magenta), and anti-GFAP antibody for astrocytes (cyan). To visualize the whole retina, a 20x Plan Apo 0.8 NA (numerical aperture) objective was used in combination with a 100-position tile scan, a 15-μm Z-stack, and 3 fluorescent channels.
Results
The Large Volume Computational Clearing (LVCC) [2,3] approach allowed a clear visualization of the interactions between endothelial cells, microglia, and astrocytes within the retina (see figure 1). Typically, these cells are difficult to discern in the retina with a widefield microscope. The high speed of acquisition with a THUNDER Imager 3D Cell Culture allowed a large tile scan, approximately 24 GB, to be acquired in minutes. The Focus Map approach with the LAS X Navigator software allowed the retina sample to remain in focus over large distances.
Conclusions
The THUNDER technology Large Volume Computational Clearing (LVCC) [2,3] significantly enhances contrast when imaging whole mouse retina enabling an efficient study of interactions between endothelial cells, blood vessels, microglia, and astrocytes when compared to conventional widefield imaging.
References
- H.J. Junge, S. Yang, J.B. Burton, K. Paes, X. Shu, D.M. French, M. Costa, D.S. Rice, W. Ye, TSPAN12 Regulates Retinal Vascular Development by Promoting Norrin- but Not Wnt-Induced FZD4/β-Catenin Signaling, Cell (2009) vol. 139, iss. 2, pp. 299-311, DOI: 10.1016/j.cell.2009.07.048.
- J. Schumacher, L. Bertrand, THUNDER Technology Note: THUNDER Imagers: How Do They Really Work? Science Lab (2019) Leica Microsystems.
- L. Felts, V. Kohli, J.M. Marr, J. Schumacher, O. Schlicker, An Introduction to Computational Clearing: A New Method to Remove Out-of-Focus Blur, Science Lab (2020) Leica Microsystems.
Related Articles
-
What are the Challenges in Neuroscience Microscopy?
eBook outlining the visualization of the nervous system using different types of microscopy…
Jun 14, 2023Read article -
Central Nervous System (CNS) Development and Activity in Organisms
This article shows how studying central nervous system (CNS) development in Drosophila-melanogaster…
May 12, 2023Read article -
Going Beyond Deconvolution
Widefield fluorescence microscopy is often used to visualize structures in life science specimens…
Mar 22, 2023Read article
Related Pages
-

THUNDER Imaging Systems
To answer important scientific questions, THUNDER Imaging Systems enable you to obtain a clear view…
Visit related page