Introduction
One of the biggest challenges of studying human brain development is accessing intact and functioning human brain tissue [1]. Recent advances in the development of 3D hiPSC-derived neural in vitro cultures have permitted more accurate modeling of in vivo tissues compared with conventional 2D monolayer neuronal cultures [1,2]. Neural spheroids are 3D iPSC-derived neuronal cultures that recapitulate the cytoarchitecture of the human brain and can be used to model brain tissue during development [1,2]. For example, neural spheroids assembled in vitro can be used to investigate how neurons migrate, mature, and integrate into functional networks in the developing cerebral cortex. Advances in the imaging and study of neural spheroids hold tremendous potential for gaining mechanistic insights into previously inaccessible aspects of neurodevelopmental disorders, developing novel therapeutics, and facilitating neural regeneration research.
Challenges
Imaging of thick 3D biological specimens, such as neural spheroids, requires an imaging solution that allows fast screening over a large area, while producing sharp images with good contrast at deep points inside the specimen. Accordingly, imaging live spheroids using widefield, camera-based fluorescence microscopy presents several important challenges. Despite its ease of use, speed, and detection sensitivity, when used to visualize thick specimens, widefield microscopy often produces images with an out-of-focus blur or “haze.” This effect arises due to detected fluorescence signals emitted from out-of-focus planes within the specimen and significantly decreases image contrast. In addition, confocal microscopy can be used to capture 3D images of spheroids, however, it often requires lengthy scan times to capture images of whole neurospheres.
Methods
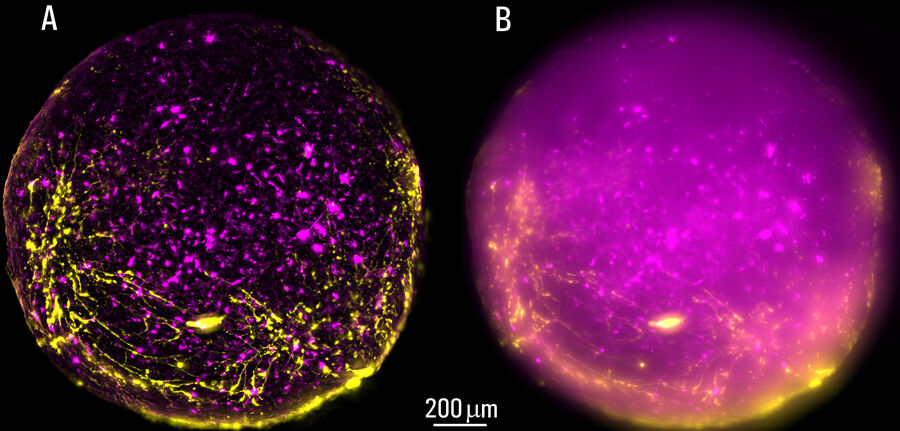
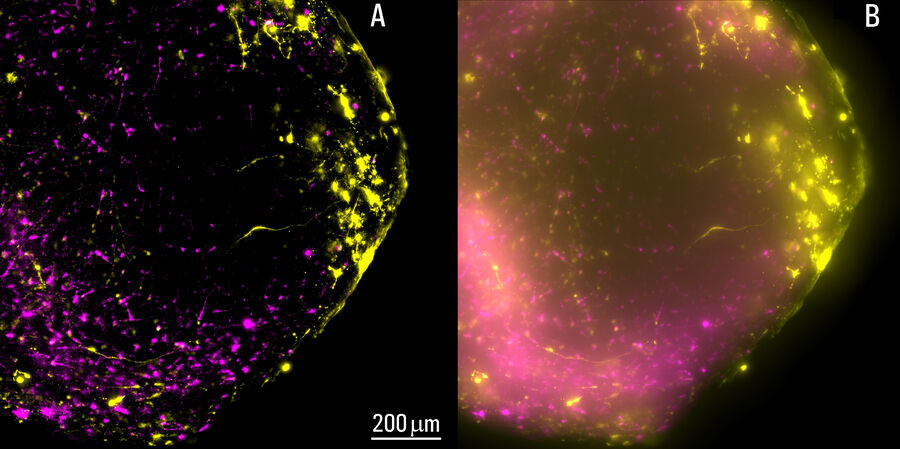
Live, intact cortical spheroids of 1.7 mm diameter were imaged with THUNDER Imager Model Organism and THUNDER Imager Tissue. Images of cortical spheroids with the THUNDER Imager Model Organism were acquired using a 2x, 0.15 NA objective and a 3.4x zoom factor. Spheroid images taken with THUNDER Imager Tissue were captured using a 10x objective which did not allow imaging of the whole neural sphere. For both THUNDER Imagers, Large Volume Computational Clearing (LVCC) [3] was applied to clear the haze and improve contrast and resolution of the original raw images.
Results
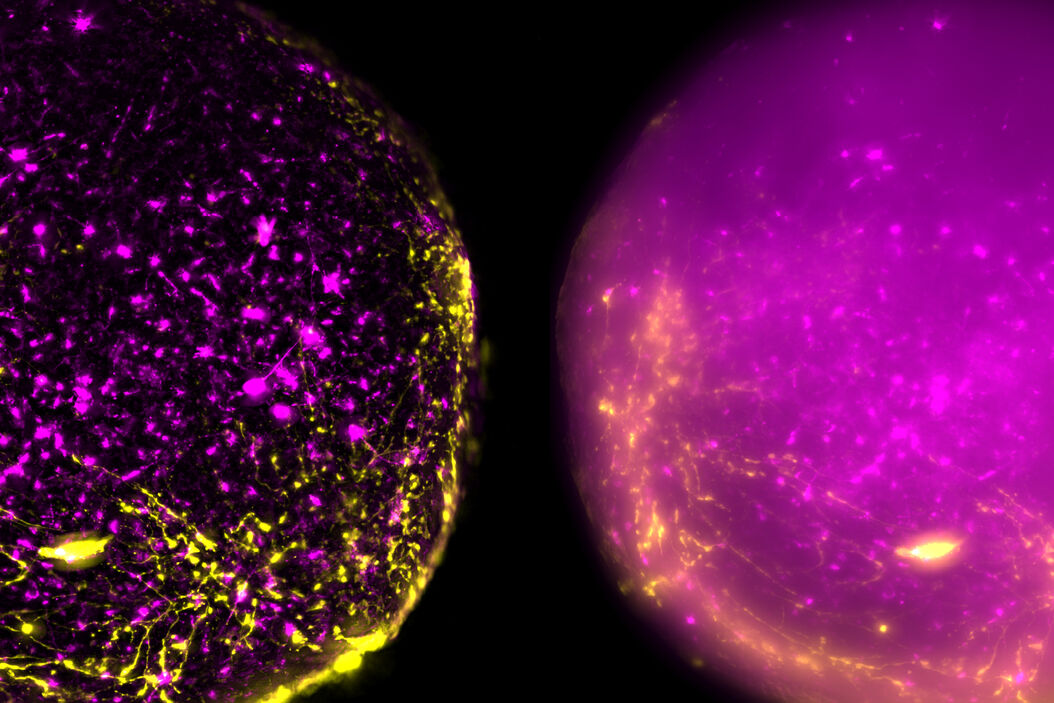
The process of imaging the cortical spheroids using a lightsheet microscope requires a significant time investment. In contrast, THUNDER Imagers provide a fast way to screen and image neural spheroids due to the faster speed of widefield stereomicroscopy, combined with the power of computational clearing . The images of cleared cortical spheroids presented in figure 1 were captured with THUNDER Imager Model Organism. Moreover, similar images of the cortical spheroids displayed in figure 2 were acquired with THUNDER Imager Tissue. While both types of THUNDER Imagers can be used for imaging of neurospheres, the THUNDER Imager Model Organism (stereo microscopy combined with computational clearing [3]) enables users to observe whole spheroids, as well as distinct regions of a spheroid, which makes specimen screening faster and easier.
Conclusions
Using a THUNDER Imager Model Organism or THUNDER Imager Tissue and Large Volume Computational Clearing (LVCC) [3] to image cortical spheroids produced sharp and clear images, while eliminating the out-of-focus blur or haze typical of widefield systems. In addition, THUNDER Imagers allowed easy screening over large areas of the spheroids with higher contrast and sharper details throughout the Z-stack used for 3D image reconstruction. For the imaging of cortical spheroids, the THUNDER Imager Model Organism (based on stereo microscopy) offers the ability to freely zoom out and in on the specimen for easy observation of whole spheroids and distinct regions. This ability allows for more rapid and straightforward screening of specimens.
References
- S.P. Paşca, Assembling human brain organoids, Science (2019) vol. 363, iss. 6423, pp. 126-127, DOI: 10.1126/science.aau5729.
- F. Birey, J. Andersen, C. Makinson, S. Islam, W. Wei, N. Huber, H.C. Fan, K.R. Cordes Metzler, G. Panagiotakos, N. Thom, N.A. O’Rourke, L.M. Steinmetz, J.A. Bernstein, J. Hallmayer, J.R. Huguenard, S.P. Paşca, Assembly of functionally integrated human forebrain spheroids, Nature (2017) vol. 545, pp. 54–59, DOI: 10.1038/nature22330.
- J. Schumacher, L. Bertrand, THUNDER Technology Note: THUNDER Imagers: How Do They Really Work? Science Lab (2019) Leica Microsystems.
Related Articles
-
What are the Challenges in Neuroscience Microscopy?
eBook outlining the visualization of the nervous system using different types of microscopy…
Jun 14, 2023Read article -
Central Nervous System (CNS) Development and Activity in Organisms
This article shows how studying central nervous system (CNS) development in Drosophila-melanogaster…
May 12, 2023Read article -
Going Beyond Deconvolution
Widefield fluorescence microscopy is often used to visualize structures in life science specimens…
Mar 22, 2023Read article
Related Pages
-
Alzheimer Plaques: fast Visualization in Thick Sections
More than 60% of all diagnosed cases of dementia are attributed to Alzheimer’s disease. Typical of…
Visit related page -
THUNDER Imagers: High Performance, Versatility and Ease-of-Use for your Everyday Imaging Workflows
This webinar will showcase the versatility and performance of THUNDER Imagers in many different life…
Visit related page -
Evaluating Axon Regeneration After Brain or Spine Trauma of Mice
Damaged nerve regeneration was investigated using mouse spinal cord sections treated with compounds…
Visit related page