Epi-Fluorescence Microscopy
Johann Sebastiaan Ploem was born in 1927 in Sawahlunto (Sumatra) as a son of a Dutch coal-mining engineer. In his early childhood he returned back to the Netherlands with his parents, where he established painting as one of his passions. Having finished high school, he discovered another fascinating area of colors as we will learn later on. Ploem decided to study medicine and was educated in Utrecht, Harvard and Amsterdam. An academic career followed during which he worked for the Universities of Miami and Amsterdam before being promoted as a professor at the medical department of Leiden, the Netherlands.
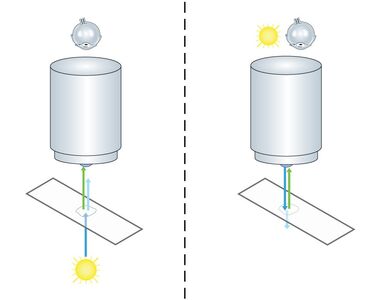
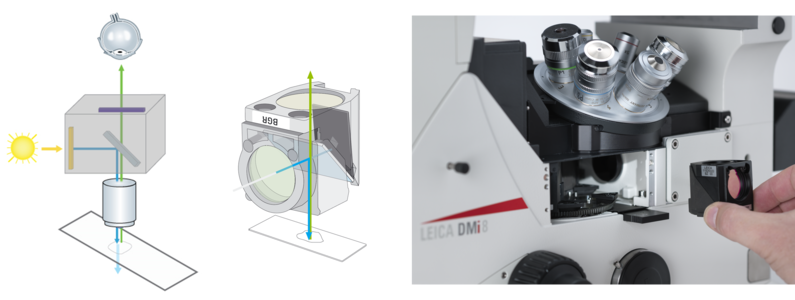
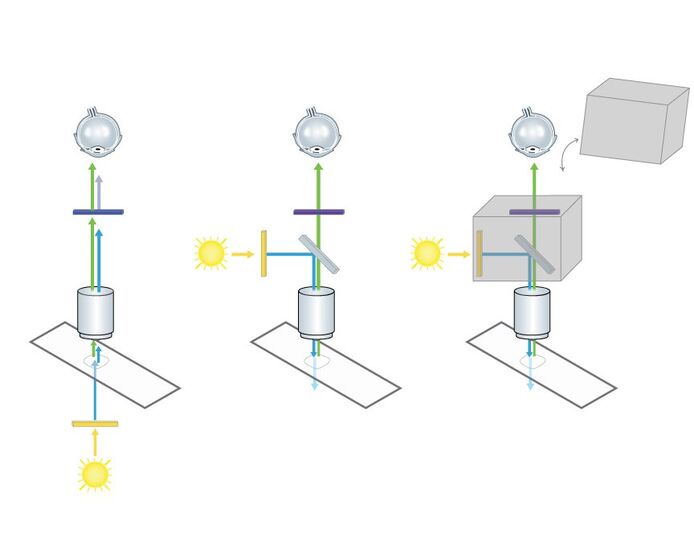
During his research activities he discovered fluorescence microscopy as a powerful tool. In the 1960s a special type of specimen illumination became popular, which was in fact already known and described in 1925 by Policard and Paillot, who were interested in autofluorescence events of the silk worm (Policard and Paillot, 1925). Some researchers restarted the two French scientists’ project to put fluorescence illumination and detection of the sample on the same side of the microscope. This principle of using incident light was called “Epi-Illumination” and stood in contrast to transmission microscopy. One big advantage of using this technique for fluorescence microscopy is to avoid the detection of emission light delivered by the light source (Fig. 1). Another benefit is of a more mechanical character: In transmitted illumination, condenser and objective have two separate optical axes which have to be aligned carefully. In epi-illumination the objective is both: a condenser and a light-collecting objective. Like this, alignment problems can be avoided.
Dichroic Beam Splitters
A very important input for fluorescence epi-illumination microscopy was given by two researchers from the Soviet Union several years earlier already. Brumberg and Krylova had developed a so called dichroic beam splitter for UV excitation with incident light (Brumberg and Krylova, 1952).
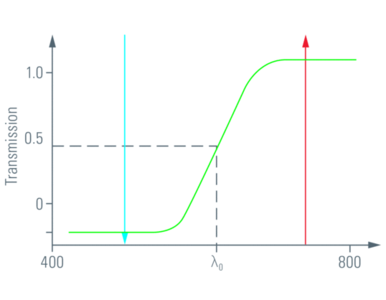
A dichroic material is able to let light of a certain wavelength range pass through, whereas light of other wavelengths is reflected (Fig. 2).
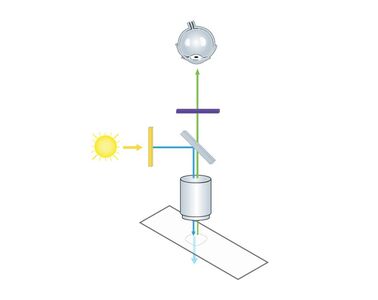
This principle is indispensable for fluorescence epi-illumination, since the excitation light has to be fused into the microscope’s light path somehow (Fig. 3). More precisely, the dichroic beam splitter is impenetrable for the wavelength of the desired excitation light coming from the light source and reflects the excitation light to the sample. Fluorescence derived from the specimen in turn can pass the dichroic beam splitter to the detection side.
Unfortunately because of the lack of information exchange across the Iron Curtain, Ploem did not know about the developments in Russia. Nevertheless he started using dichroic beam splitters himself. In the special case of Ploem, he developed a beam splitter together with the well known producer for specialty glasses Schott (Mainz), which reflected blue and green light (Ploem, 1965). When he modified an "Opak" epi-illuminator with a neutral beam splitter contributed by the Leitz company afterwards, by introducing a slider with four different dichroic beam splitters he could achieve the very fast and easy change of excitation light wavelength between UV, violet, blue and green (Ploem, 1967) (Fig. 4).
The Fluorescence Filter Cube
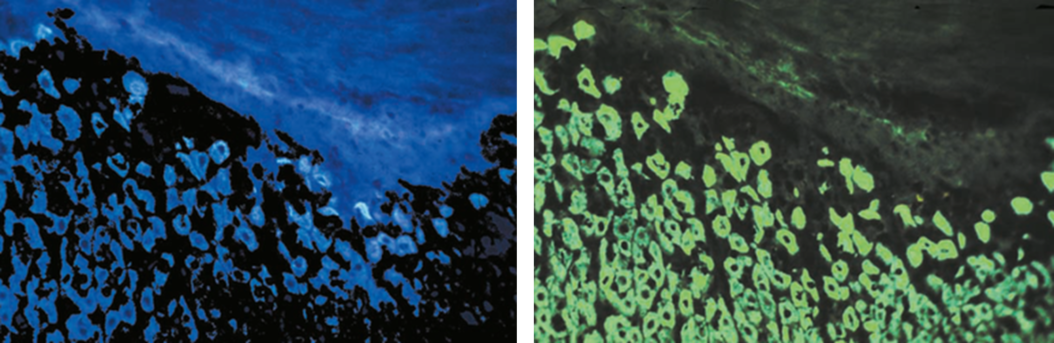
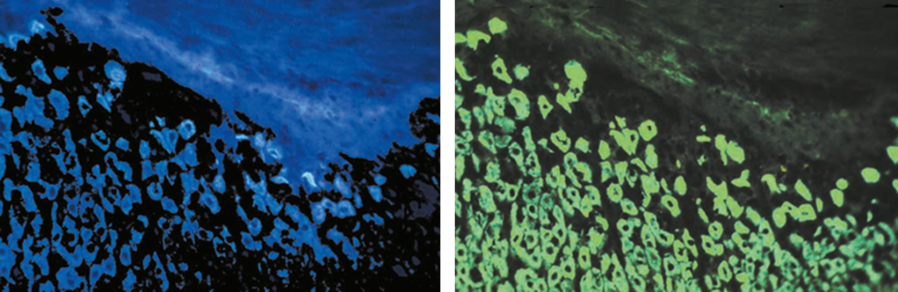
The development of dichroic beam splitters to create excitation light with discrete wavelengths had an important advantage. Excitation in the UV-light spectrum (~100 nm – 380 nm) was very common at that time but had an annoying side effect: Autofluorescence. A lot of tissue substances are excited by UV-light, resulting in a faint background glowing (Fig. 5). By fitting the dichroic mirrors’ reflection wavelengths to a green or blue range, Ploem was able to reach the excitation maxima of the two fluorescent dyes FITC (494 nm) and TRITC (541 nm), which were used very commonly at those times, without producing autofluorecence. FITC (Fluorescein isothiocyanate) and TRITC (Tetramethylrhodamine-5-(and 6)-isothiocyanate) can be coupled to antibodies and are still used in immunofluorescence microscopy. By hitting their excitation maxima in a small range, the contrast of tissue specimen was enhanced markedly (Fig. 5). The excitation light beam produced with Ploem’s dichroic beam splitters was fitted to the excitation maximum of FITC so efficiently that even light sources with a rather bad emission spectrum could be used.
Considering these facts it was now possible to use the advantages of epi-illumination by applying narrow band excitation with blue and green, powered with ordinary high-pressure mercury arc lamps. This enhancement satisfied the upcoming demand for fluorescence microscopy in life sciences and medicine.
Out of Ploem’s inventions, Leitz designed a new multi-wavelength fluorescence epi-illuminator with four rotating dichroic beam splitters which could excite specimen in the range of UV, violet, blue and green light: the Leitz PLOEMOPAK.
Further achievements were done by the Leitz employee W. Kraft, who combined a dichroic beam splitter together with the adequate excitation and emission filters in one work piece, a so called filter cube or filter block (Kraft, 1969 und Kraft, 1972) (Fig. 6). His investigations resulted in the design of an epi-illuminator with several sets of four of these filter cubes which are still the basis of nearly every multi-wavelength fluorescence microscope today.
Summary
With the technical fundament built up by Ploem and his contemporaries and successors, we enjoy today the privilege of watching countless different fluorophores with the adequate filter cube put into the epi-illuminator (Fig. 7). Researchers can even build their own cubes to customize excitation and emission parameters to their needs.
Due to the automation of modern research microscopes, switching filter cubes during an experiment is not more than a click of a button. Scientists can toggle between different fluorophores in a split second and thus can watch even living specimen fluorescently marked with different reporters.
References
- Brumberg, E. M., Krylova, T. N.: O fluoreschentnykh mikroskopopak. Zh. obshch. biol. 14, 461, 1953.
- Ploem, J. S.: Die Möglichkeit der Auflichtfluoreszenzmethoden bei Untersuchungen von Zellen in Durchströmungskammern und Leightonröhren. Xth Symposium d. Gesellschaft f. Histochemie, 1965. Acta Histochem. Suppl. 7, 339–343, 1967.
- Ploem, J. S.: The use of a vertical illuminator with interchangeable dichroic mirrors for Fluorescence microscopy with incident light. Zeitschr. f. wiss. Mikroskopie 68, 129–142, 1967.
- Kraft, W.: Die Technologie des Fluoreszenzopak, Leitz Mitt. Wiss. u. Techn. IV/6, 239–242, 1969.
- Kraft, W.: Fluorescence Microscopy and Instrument Requirements. Leitz Mitt. Wiss. u. Techn. V/7, 193–206, 1972.
- Policard, A., Paillot, A.: Etude de la sécrétion de la soie à I'aide des rayons ultraviolets filtrés (lumière de Wood). Comptes Rendus de l'Académie des Sciences Paris 181, 378–380, 1925.
<link science-lab multi-wavelength-epi-illumination-in-fluorescence-microscopy>www.leica-microsystems.com/science-lab/multi-wavelength-epi-illumination-in-fluorescence-microscopy