Historical background
Beginning with Luigi Galvani’s pioneering work in the 18th century and the work of Emil du Bois-Reymond, Johannes Peter Müller, and Hermann von Helmholtz in the 19th century, the excitability of membranes and cells has always been of major interest for research on the nervous system [1]. Alan Lloyd Hodgkin and Andrew Huxley revealed the ion channel events of action potentials in 1952 using the voltage-clamp technique and were awarded the Nobel Prize in Physiology and Medicine in 1963 for their outstanding work [2,3].
At this time, voltage clamping could only be applied to rather big cells as sharp microelectrodes were needed to penetrate the membrane. In the late 1970s, Bert Sakmann and Erwin Neher refined the voltage-clamp technique and, for the first time, resolved single ion channel currents across a membrane patch of a frog skeletal muscle. They were also honored with the Nobel Prize in Physiology and Medicine in 1991 [4,5]. The next breakthrough was the invention of the giga seal by Bert Sakmann in 1980 which immensely improved the signal-to-noise ratio and allowed the recording of even smaller currents [6].
Electrophysiology, pioneered in special biophysical laboratories, now expanded to basic biological and medical research and became one of the most important tools for the investigation of the behavior of single cells or whole cellular networks in the nervous system.
General principle of the patch-clamp technique
The patch-clamp technique allows the investigation of a small set or even single ion channels [7]. Thus, it is of special interest in the research of excitable cells, such as neurons, cardiomyocytes, and muscle fibers [8].
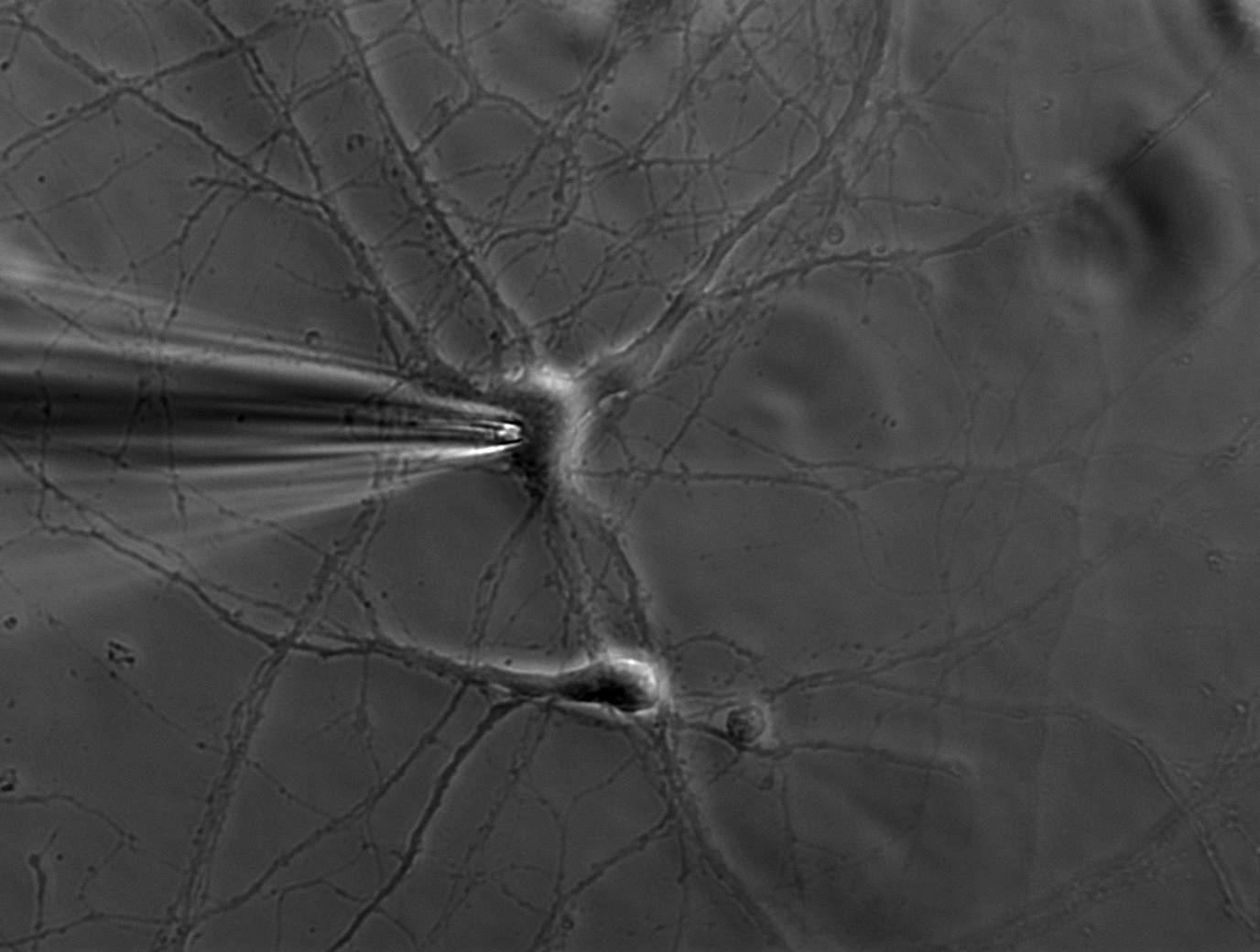
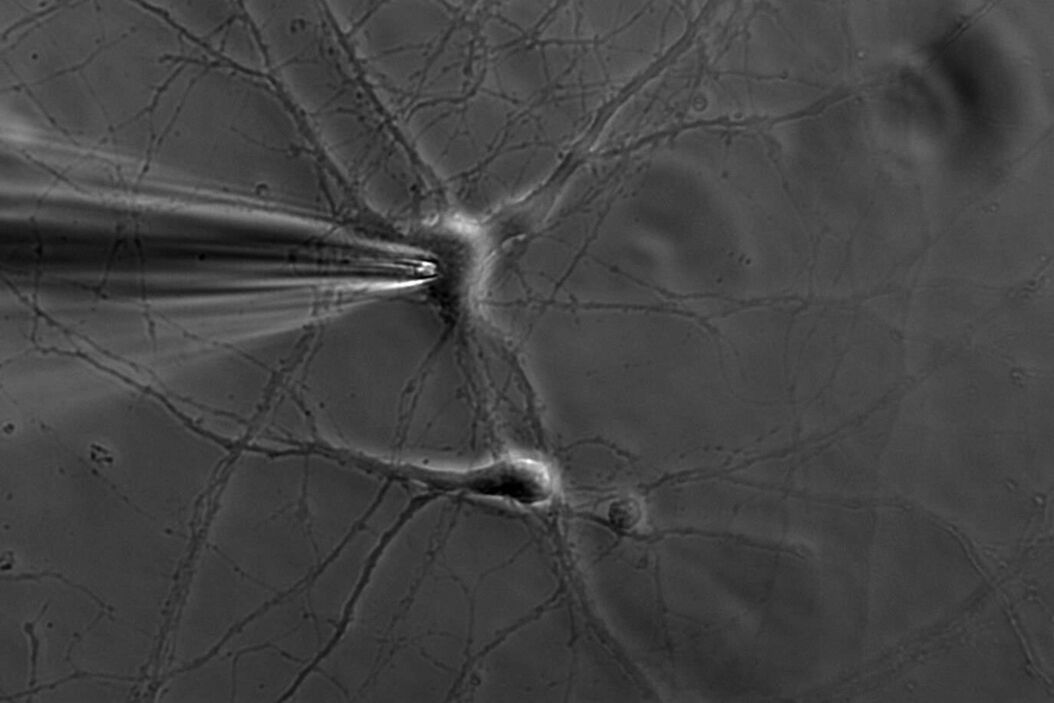
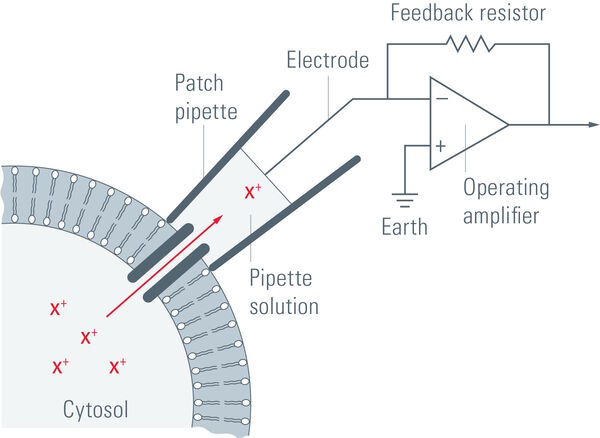
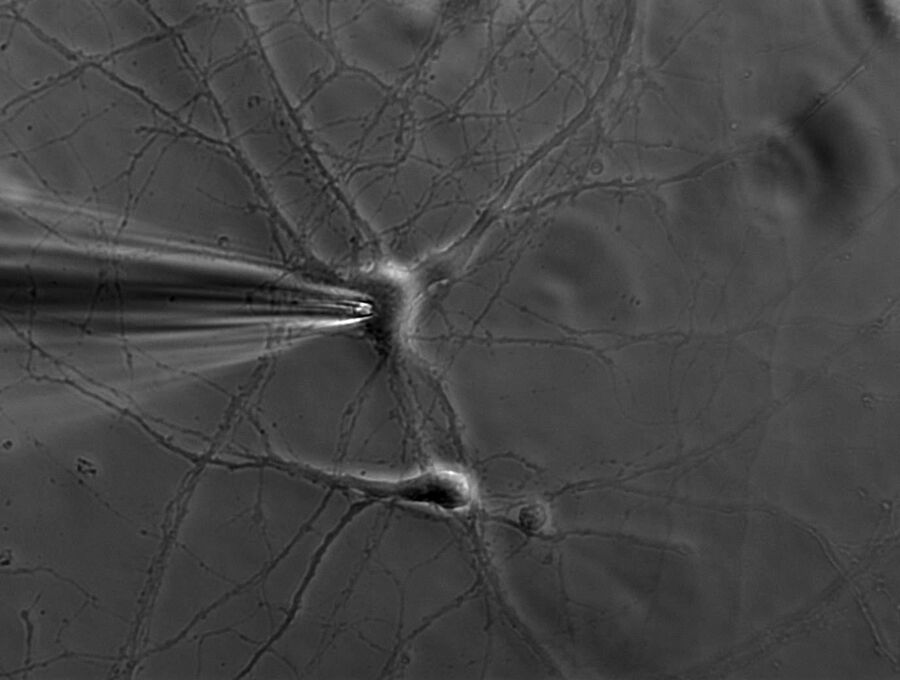
A single ion channel conducts around 10 million ions per second. Yet the current is only a few picoamperes. Recording currents in this order of magnitude is quite challenging not only for the researcher, but also for the equipment. In principle, thin glass or quartz pipettes with a blunt end are sealed onto the membrane (refer to figure 1, 2, and 3).
Suction is applied to aid the development of a high-resistance seal in the gigaohm range. This tight seal electrically isolates the membrane patch, which means that all ions flowing through the membrane patch flow into the pipette and are recorded by a chlorided silver wire electrode (acts as a silver chloride (Ag/AgCl) electrode) connected to a highly sensitive electronic amplifier. A bath electrode is used to set the zero level.
To prevent alterations in the membrane potential, a compensating current that resembles the current that is flowing through the membrane is generated by the amplifier as a negative feedback mechanism (refer to figure 1).
The membrane potential of the cell is measured and compared to the command potential. If there are differences between the command potential and the measurement, a current will be injected. This compensation current will be recorded and it allows conclusions about the membrane conductance to be made. The membrane potential can be manipulated independently of ionic currents and this ability allows investigation of the current-voltage relationships of membrane channels.
Patch-clamp configurations
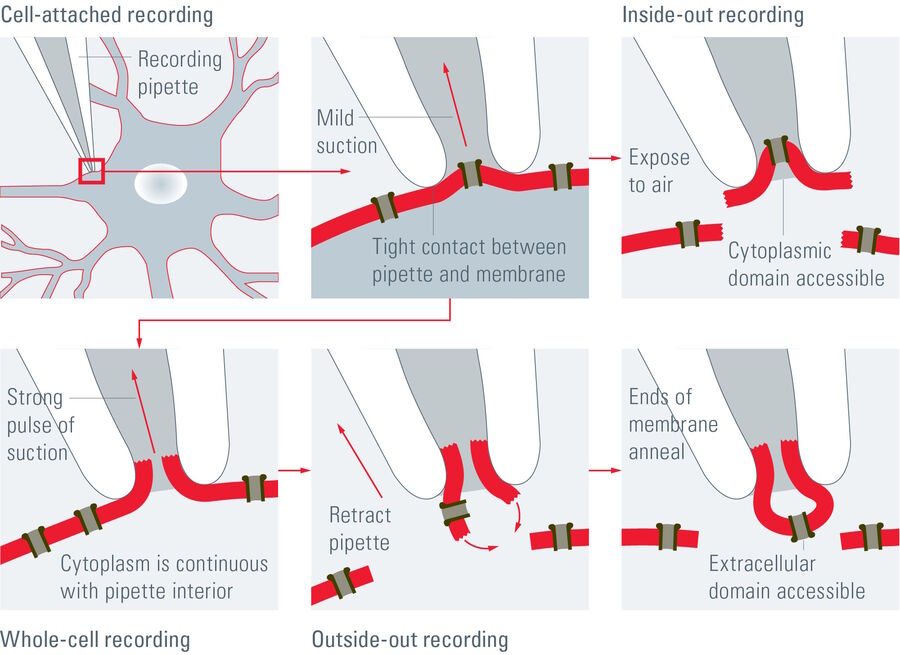
Depending on the research interest, different configurations can be used. In the cell-attached mode, the membrane patch is left intact (refer to figure 3). A modification of the cell-attached mode is the loose-patch. In this case, the pipette is not tightly sealed to the membrane, but is only loosely attached to it. This mode is often used to record action potentials in neuronal cells. The advantage is that the composition of the cytoplasm is not influenced. On the other hand, however, the intracellular environment cannot be controlled.
Applying pore-forming agents (usually antibiotics) via the patch pipette results in a perforated patch which guarantees ionic continuity, but assures that intracellular proteins are not washed out by the pipette solution. The most commonly used patch-clamp mode is the whole-cell mode (refer to figure 3). To achieve this mode, the membrane patch is disrupted by briefly applying strong suction. The interior of the pipette becomes continuous with the cytoplasm. This method is used to record the electrical potentials and currents from the entire cell. For whole-cell measurements, the researcher can choose between two configurations: the voltage-clamp mode where the voltage is kept constant and current is recorded or the current-clamp mode where the current is kept constant and changes in the membrane potential can be observed.
Moreover, it is also possible to record currents only from a small patch instead of the whole cell. This raises the chances of recording single channels. The patch can be orientated in two different directions inside the patch pipette. To achieve the inside-out configuration the patch pipette is attached to the cell membrane and is then retracted to break off a patch of membrane (Figure 3). In this case the cytosolic surface of the membrane is exposed. This is often used to investigate single channel activity with the advantage that the medium which is exposed to the intracellular surface can be modified.
If the aim is to study the influence of extracellular cues, such as neurotransmitters, the outside-out configuration (refer to figure 3) should be chosen. In this case, the pipette is retracted during the whole-cell configuration, causing a rupture and rearrangement of the membrane. In this configuration, the extracellular surface is exposed and, thus, extracellular cues can easily be applied.
Requirements
Patch-clamp experiments can either be performed on cultured cells, acutely dissociated cells, or acute vibratome slices which allow investigation of the cell’s electrophysiological properties in their natural environment. The ion channel of interest can also be isolated and expressed heterogeneously in a common cell line (e.g. HEK293, CHO, LNCaP).
Depending on the sample, either an inverted (cultured cells) or an upright fixed stage microscope (for slices) with a stable platform is needed. If cells in acute slices are investigated, an infrared DIC (differential interference contrast) [9] is recommended to visualize the membrane more easily. The microscope should be placed on an anti-vibration table, because any movement could be fatal to the seal between the pipette and the membrane.
A micromanipulator is needed to move the pipette precisely. Very fine pipettes are formed by heating and pulling small glass or quartz capillary tubes. The diameter of the pipette tip is around 1 µm which encloses a membrane patch that contains only a few or even just one ion channel. The tip of the pipette is then heat-polished in a microforge so that a high-resistance seal can be attained once the tip is in contact with the membrane. The pipette is filled with a solution that resembles either the extracellular solution or the cytoplasm, depending on the recording mode. The pipette is mounted on a micromanipulator to permit precise movements towards the cell membrane.
For detecting the current, a chlorided silver wire is used. The bath electrode, which is also a chlorided silver wire (acting as a Ag/AgCl electrode), sets the zero current value. A differential amplifier with a low-noise transistor is connected to a computer for data acquisition and digitization. Specific software can be purchased to control the amplifier and analyze the data. Alternatively, an oscilloscope can be used to monitor the currents. If desired, a perfusion system can be added to the setup. Substances can either be applied via a perfusion pencil or by using a POC (perfusion open and closed) chamber.
Applications of patch clamping
Patch-clamp experiments are used to gather data which can answer a large variety of physiological questions, not only in the field of neuroscience. During the last three decades, patch-clamp recordings have also become more important for the investigation of ion channels in non-excitable cells. It is also a very important method for medical research, because many diseases are related to a malfunction of specific ion channels. In pharmacological research, automated patch clamping is used to screen potent substances for ion-channel modifications.
Patch-clamp recordings can also be combined with live-cell imaging approaches, such as calcium-ion (Ca2+) imaging. In this case, a Ca2+-sensitive fluorescent dye is applied to the cell via the patch pipette. The membrane current and changes in fluorescence are recorded simultaneously. Similar experiments can be performed with dyes which are sensitive to pH or chlorine (Cl).
Video: Preparation of acute brain slices
Video: Electrophysiology on acute brain slices
Video: Electrophysiology on cultured cells
References
- S.M. Schuetze, The discovery of the action potential, Trends in Neurosciences (1983) vol. 6, pp. 164-168, DOI: 10.1016/0166-2236(83)90078-4.
- A.L. Hodgkin, The ionic basis of nervous conduction, Nobel Lecture, Nobel Prize in Physiology or Medicine 1963, Nobel Prize Organization (2023).
- A.F. Huxley, The quantitative analysis of excitation and conduction in nerve, Nobel Lecture, Nobel Prize in Physiology or Medicine 1963, Nobel Prize Organization (2023).
- B. Sakmann, Elementary Steps in Synaptic Transmission Revealed by Currents through Single Ion Channels, Nobel Lecture, Nobel Prize in Physiology or Medicine 1991, Nobel Prize Organization (2023).
- E. Neher, Ion Channels for Communication Between and Within Cells, Nobel Lecture, Nobel Prize in Physiology or Medicine 1991, Nobel Prize Organization (2023).
- O.P. Hamill, A. Marty, E. Neher, B. Sakmann, F.J. Sigworth, Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches, Pflugers Archiv (1981) vol. 391, iss. 2, pp. 85–100, DOI: 10.1007/BF00656997.
- A. Molleman, Patch clamping: an introductory guide to patch clamp electrophysiology (John Wiley & Sons, Ltd, West Sussex, England, 2003) DOI: 10.1002/0470856521.
- B. Hille, Ionic Channels of Excitable Membranes (Sinauer Associates, Inc., 2001) ISBN: 9780878933211.
- W. Ockenga, J. DeRose, C. Greb, Differential Interference Contrast (DIC) Microscopy: Making visible unstained, transparent structures of biological specimens which are difficult to see with brighfield illumination, Science Lab (2023) Leica Microsystems.