What to expect
Key Learnings
- Discover how to use antibodies for multiplexed imaging
- Learn the basics of panel design for open multiplexing
- Understand how to choose and source antibodies to save money and time
What are antibodies?
Antibodies are immune molecules produced by a class of immune cells known as B lymphocytes. Unique antibody genes are generated through a complex process of genetic rearrangements that occur within B lymphocytes. Once expressed into proteins, they are selected and matured based on their ability to bind to antigen proteins of interest, such as a bacterial cell wall protein, and fulfill a wide variety of downstream immune functions.
Antibodies are used in a wide variety of biological experiments and contexts because they can be directed against specific proteins of experimental interest. Even more conveniently, alongside polyclonal antibodies produced by the immune systems of animals and human beings, scientists have also developed highly specific monoclonal antibodies that bind only one specific epitope (or protein shape) of a given antigen.
Antibodies can be used experimentally to detect proteins on an electrophoretic gel (immunoblotting). In addition, they can be deployed in color changing antigen recognition assays known as ELISAs (enzyme-linked immunosorbent assays) or can be directly conjugated to dyes and enzymes and used directly to stain a protein of interest in tissue. It is this last function, and other closely related methods, that has seen wide use in both traditional immunohistochemistry (IHC) and multiplexed imaging.
How are antibodies used in multiplexed imaging?
Owing to the binding specificity of antibodies, many different proteins can be individually bound by antibodies. In the past, the obstacle to visualization of more markers for imaging experiments was imposed by the limited number of available fluorescent channels that can be practically used by a given microscope. This remains the main obstacle that multiplexed techniques surmount to achieve higher numbers of visualized markers.
Leica Microsystems’s Cell DIVE multiplexed imaging platform, for example, uses cyclical antibody staining using dye-conjugated antibodies followed by inactivation of those dyes to repeatedly probe the sample and thus image more biomarkers. However, alternative approaches exist, such as using antibodies with barcoded tags that bind fluorophores only when a dye with a complementary barcode is applied. In all cases, the specific binding of the antibody to a given antigen is leveraged to image the underlying proteins.
Choosing antibodies for multiplexed imaging
The antibodies utilized for a given application will differ depending on the needs of that application. For example, antibodies that recognize proteins effectively in immunoblots may not be equally effective for immunohistochemistry. Certain multiplexed imaging techniques may also impose further restrictions on antibodies, such as having to tolerate certain tags or dye conjugations. It is important to thoroughly investigate the multiplexed method of choice and select only those antibodies that meet the requirements of the method.
The choice of sample type and preparation will also influence antibody selection. For example, when working with human tissue, multiple preparations such as formalin-fixed paraffin-embedded tissue (FFPE) or unfixed frozen tissue are available. It is not uncommon that antibodies that work well on frozen tissue do not work well on FFPE and vice-versa. In addition, as mentioned above, both monoclonal and polyclonal antibodies are available. In general, monoclonal antibodies are preferred owing to their more specific antigen binding profile and reduced off-target interactions.
For an open multiplexed imaging methodology like Cell DIVE , for example, antibodies of first choice are those previously validated in FFPE tissue and suitable for IHC applications.
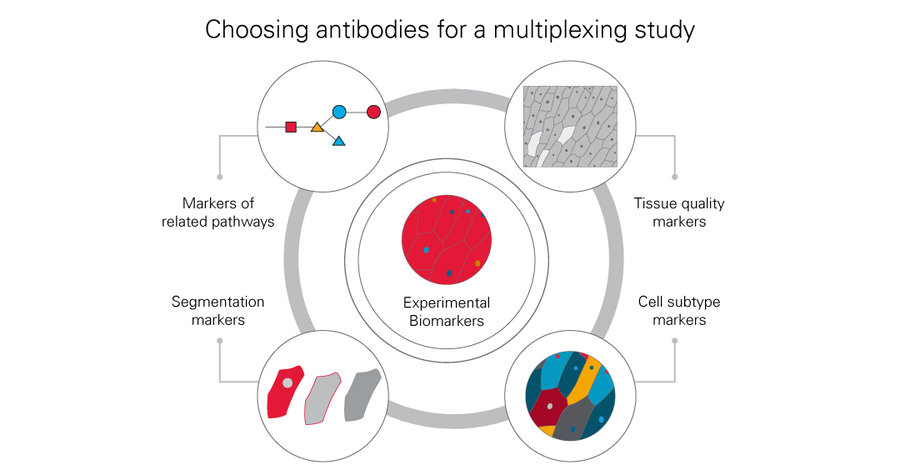
Choosing biomarker targets for studies
Multiplexed imaging opens the door to the use of many more biomarkers in a study than would be otherwise be practical using a traditional imaging approach. The “budget” of available antibodies is therefore much larger. However, care should be taken in the selection of antibodies to ensure the highest return on investment for the time and effort spent on an experiment.
The experimental biomarkers of immediate interest should first be considered. It may also be useful to identify biomarkers in similar or related biological pathways that may flesh out the understanding of an experiment. The ultimate method of image analysis should also be considered. If automated image analysis through image segmentation is employed, a variety of markers can be included in the study to outline cell compartments and tissues more clearly. For example, several markers could be included in the study to assist in the complete segmentation of important biological structures such as blood vessels. It may also be useful to include biomarkers with broad staining patterns to assess tissue quality throughout the length of the study. Biomarkers that more clearly identify cellular subtypes, such as immune cell types, should also be considered to add depth to the study.
Sourcing antibodies
For a variety of multiplexed imaging techniques, antibodies may most easily be sourced in panels or kits directly from the manufacturer of the instrument. It may also be possible to acquire antibodies separately from the device manufacturer and customize them to work with the system of choice. For Cell DIVE, antibodies are sourced by the user from antibody companies of their choice.
The manufacturer of the imaging system may also provide a list of pre-validated antibodies, such as in the case of Cell DIVE’s Validated Antibody List . To save time and validation costs, antibodies should be preferentially selected from such a list. In systems that make use of dye-conjugated antibodies, selecting commercially conjugated, validated antibodies, may offer additional savings in labor.
If an antibody of choice is not available on a validated list, it may be advisable to carefully consult that list and ensure that closely related antibodies are not available. If, for example, an antibody targeting a biomarker in the same biological pathway as the first-choice antibody is available on the list while the first choice is not, it may be advantageous to select the antibody on the list.
In such cases where a compatible antibody is not available on a validated list, the user may need to validate the antibodies themselves (see below). In this case, a small panel (3-5) of candidate antibodies should be sourced. These candidates should ideally have some literature backing showing efficacy in IHC in the sample type of interest. Antibody databases such as citeab.com can be used to accelerate this process. If antibodies will be dye -conjugated, sourcing antibodies without additives, such as preservatives, will speed the overall workflow.
Antibody validation
When suitable antibodies are acquired, they should be purified, if necessary, conjugated to dyes or prepared as is appropriate for the multiplexing methodology employed, and rigorously validated before use to ensure proper specificity and sensitivity of biomarker staining. Cell DIVE antibody validation, for example, uses a three-step characterization process . First, basic primary-secondary staining of the candidate antibody panel should be performed to assess basic staining quality. Second, successful candidates are conjugated in multiple dye-to-protein ratios (D:P) and then exposed to tissue in different concentrations. The most successful conditions are selected. Third, as Cell DIVE uses a cyclical staining and dye inactivation process, the tolerance of the target antigen to multiple rounds of that process is tested. At every step, staining is compared to literature references and in-house staining data to ensure staining quality.
Once proper validation is performed, users can be confident that the selected antibody clone and staining conditions are satisfactory for their imaging system. However, occasional testing of staining quality should be performed when antibody vendors change, or when new batches of primary antibody are acquired.
Considerations for staining
The actual method of antibody staining is often as simple as adding the antibodies to the tissue at room temperature and incubating before washing and proceeding with the experiment. However, there are several points to be considered to determine how tissue is ultimately stained with prepared antibodies.
Staining order
When working with 10, 20, or 100 biomarkers in a multiplexed imaging study, intelligently prioritizing how and when antibodies are stained and imaged is vitally important. Priority should be given to critical experimental biomarkers that directly address the hypothesis of the study. Following that, segmentation markers should be prioritized to ensure that analysis pipelines will operate smoothly. Biomarkers that suffer antigen effects in an iterative method (see above) should also be stained and imaged at appropriate rounds. Once the order is determined, it should be carried out as planned for all samples in an experimental cohort to ensure that quantitative readings of fluorescence can be directly compared.
Choosing channels
Care should be given to choosing the proper dye and fluorescent channel for a given biomarker. While there are few hard and fast rules, remember that tissue autofluorescence often differs depending on the channel used for imaging. Tissue autofluorescence is often brightest in the green channel (FITC, Cy2, etc.), and thus, only the brightest biomarkers should be used with dyes visible in this range. Conversely, tissue is often least autofluorescent in the far-red (Cy5) or near-infrared (Cy7) channels and thus biomarkers and antibodies with weak signal can be considered for those channels. Consider the performance of your antibodies during validation and plan round-based multiplexing methods accordingly.
Leaching
Some antibody-antigen pairs suffer from a loss of affinity over time. This can manifest as dye-bound antibody leaching into mountant and degrading image quality by increasing background. Leaching behavior can be assessed during antibody validation. If identified, two remedial approaches can be taken. First, antibodies prone to leaching should be imaged in channels with low autofluorescence as signal may be lost over time as leaching occurs. Second, the time between staining and imaging should be minimized by optimization of the overall imaging workflow.
Primary secondary pairs
Some multiplexed techniques, such as Cell DIVE, permit a small number of primary-secondary pairs to be used in a study. These should be prioritized to the first round and planned with careful observation of cross-species compatibility in mind. Following binding of the secondary antibody, it is often necessary to block the tissue to prevent cross-reactivity of the secondary with incoming antibody in later rounds. While not ideal for a technique that favors dye-conjugated primary antibodies, primary-secondary pairs can be a useful way to include antibodies with conjugation issues and expand the overall panel.
Crowding agents
Crowding agents, like the polymer dextran, can be added to staining solutions to improve antibody binding for low abundance antigens that can be challenging to detect. These reagents can increase local concentrations of antibody and facilitate the antigen-antibody reaction. Care should be taken to test the overall concentration of the chosen crowding agent within the staining cocktail to ensure proper behavior of the antibodies. Additionally, all antibodies included in a staining cocktail that contains a crowding agent should be carefully tested to ensure that antibody activity is not adversely affected.
Conclusion
Identifying, sourcing, testing, and using antibodies for a multiplexed imaging study is a complex and often challenging process. Careful study design and validation processes will greatly aid the researcher in reliable, robust experiments. We hope this brief guide has provided useful information and will accelerate the planning process for your next experiment. For additional information and training, please reach out to a local Leica Microsystems representative to learn more about options for multiplexing and how to achieve results for your group.