STELLARIS DLS Digital Light Sheet Microscope
Light Sheet Re-Imagined
O STELLARIS LightSheet (DLS) reúne em um só lugar um sistema confocal e um microscópio de chapa de luz, uma combinação única que visa tornar sua pesquisa mais versátil. O exclusivo design vertical do DLS, possibilitado pelos espelhos TwinFlect de propriedade da Leica Microsystems, permite combinar imagens confocais e de chapa de luz no mesmo sistema, para que você possa adaptar facilmente o método de microscopia às suas necessidades experimentais.
A DLS também traz flexibilidade à sua pesquisa com a capacidade de gerar imagens de diferentes tipos de amostras, como organismos modelo, organoides ou tecido limpo, e a capacidade de aproveitar todo o espectro de excitação. Essa flexibilidade é possível graças ao laser de luz branca STELLARIS e à capacidade de realizar experimentos com folhas de luz em várias posições, usando placas de fundo de vidro padrão. Tudo isso o ajuda a acrescentar novas e melhores maneiras de explorar suas questões de pesquisa.
Experience the power of fast and gentle volumetric imaging.
Obtain more physiologically relevant data.
DLS and STELLARIS enable even gentler imaging giving you the ability to perform fast and gentle volumetric light sheet imaging and to improve your live cell imaging applications by increasing cell viability thanks to:
- single plane illumination
- fast imaging with a sensitive sCMOS camera
- significantly increased spectral possibilities and the ability to perform even gentler imaging using excitation wavelengths in the far-red spectrum
- capacity to generate a light sheet with the resonant scanner which results in shorter pixel dwell times and, consequently, less phototoxic effects.
- obtain light sheet results with improved contrast and signal-to-noise ratio thanks to the LIGHTNING information extraction solution for DLS data.
Light sheet with confocal benefits
Thanks to the seamless integration of DLS, your light sheet imaging benefits from the technical innovations of your STELLARIS system.
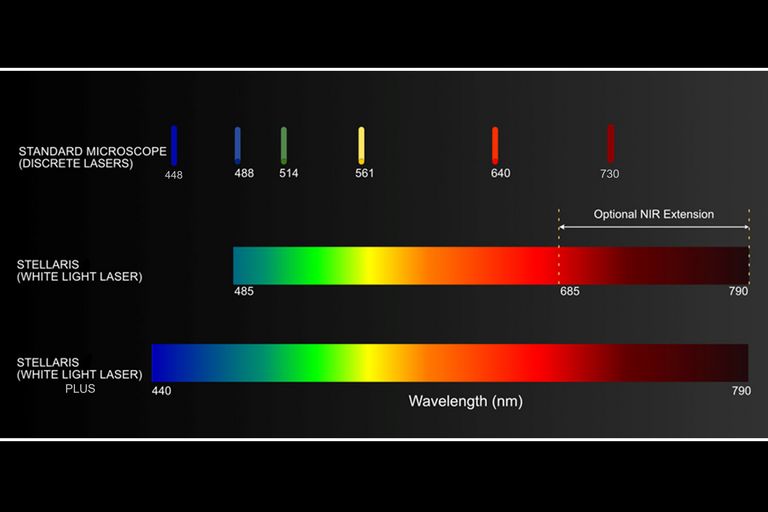
Always the right laser
All visible lasers of your STELLARIS confocal are ready to be used for light sheet imaging. There is much flexibility when choosing the right dye for your light sheet experiments with the optional diode laser lines as well as the STELLARIS next generation White Light Lasers. You can now even unlock the imaging of near-infrared dyes.
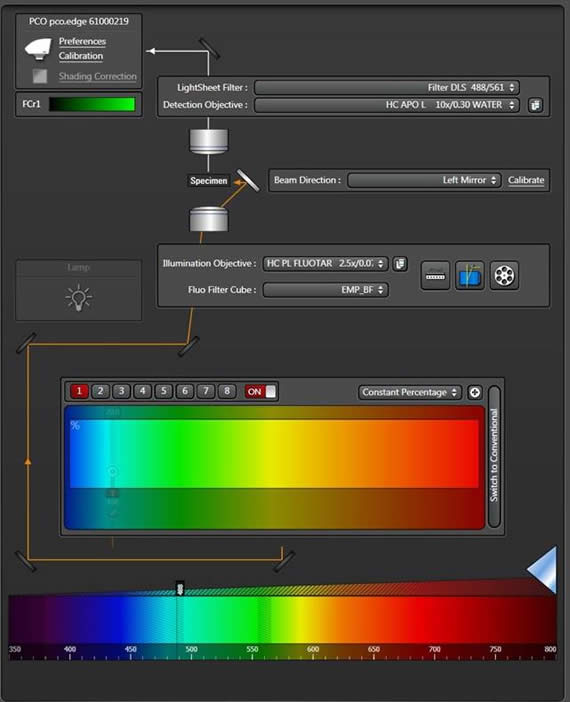
Always the right scanner
On STELLARIS systems equipped with a tandem scanner, you can choose between the resonant scanner or the field-of-view scanner (at 1400Hz) to generate the scanned light sheet. Generating the light sheet with the resonant scanner produces shorter pixel dwell times, which enables even more gentle imaging.
Enhance the potential of your research with a system that adapts to your needs
Experience the flexibility to image different types of samples.
- Image living samples and cleared specimens, such as organoids, tissue, or whole-developing organisms, in the same system and without difficult hardware changes
- Easy exchange of a growing number of detection objectives and TwinFlect mirrors to shape the light sheet depending on your needs
- DLS objectives cover water-based to organic clearing reagents
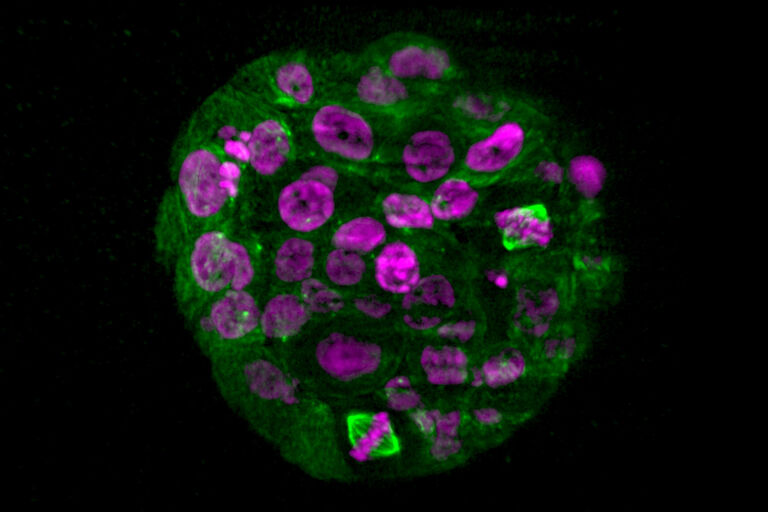
Manipulate your specimen using confocal technology
Our light sheet module is more than a functional add-on to your confocal. STELLARIS and DLS synergize to expand the options for your research. For example, you can manipulate your specimens using the confocal technology and then image with DLS.
It is simply done by switching between confocal and light sheet mode in the LAS X software. This way, photoconversion or wounding experiments with subsequent gentle long-term observations become easy and convenient.
Straightforward sample manipulation
- Easy access to your specimen to perform drug treatments
- Ability to manipulate your specimens using the confocal technology for photoconversion and wounding experiments followed by gentle and fast DLS imaging
Increase productivity of your light sheet experiments
Keep your workflow and sample handling.
- Easy incorporation of your specimens into your light sheet experimental workflow thanks to DLS unique Twinflect design.
- Transition between confocal and light sheet experiments without the need of additional cumbersome experimental setups.
- Keep the sample preparation you are familiar with
- mount and image several samples with multi-positioning experiments.
- Perform a tile scan of very large samples by using DLS in combination with the confocal system stage automation.
- Switch easily between fluorescence and widefield imaging for convenient sample navigation.
- Provide cellular and organismal context for fluorescence optical sections by using the widefield mode for acquisitions.
Workflow-oriented software design
The LAS X software guides users step by step through data recording and evaluation. The workflow-oriented design helps you to use the instrument more efficiently. A convenient calibration routine establishes the light sheet precisely.
Dual-sided illumination of the sample comes by design: each of the two opposing mirrors of the TwinFlect can be targeted by the scanner to overcome darker areas. For crisp images with a large field of view, you can merge the two images using the online or offline fusion option of the LightSheet Wizard in the LAS X software.
Tailor LAS X to your needs with additional software packages. The LAS X 3D Visualization module provides novel ways to interact with your 3D data by intuitive clipping, fast rendering, and stereo display. Tile scanning experiments enable you to observe large areas. Mark & Find experiments allow you to observe several regions of interest in a multi-position set up.
Conduct and document long-term observations
Imaging requires light, but too much light can damage your cells. Light sheet microscopy is the most gentle imaging method to date, as it reduces the overall photodamage from phototoxicity and bleaching. This automatically increases the viability of your specimen.
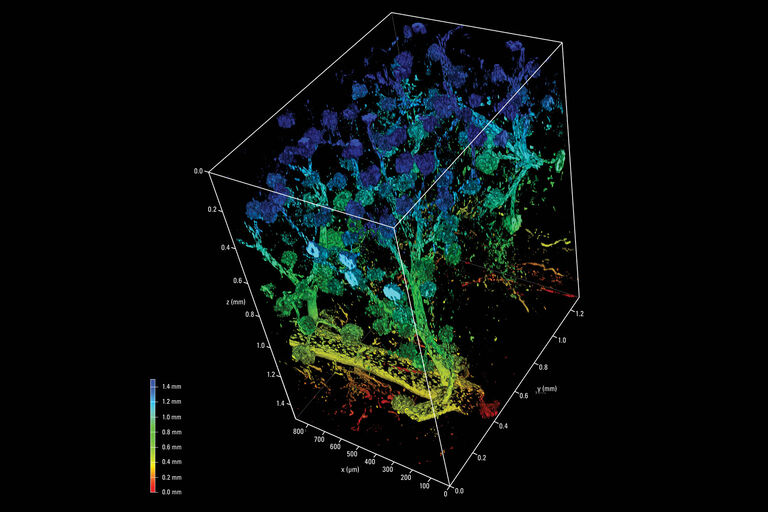
Particularly developmental biology benefits from light sheet imaging. The combination of low light illumination and high-speed acquisition allows you to follow sensitive, developing organisms like a Drosophila embryo over long periods of time and to understand how tissue and organs form in real time and 3D.