Crystalline defects
Though silicate glass can differ greatly in its composition and properties, possible defects are of similar types and causes. In addition to gaseous inclusions (bubbles), crystalline glass defects are common in everyday production. Quick defect identification is critical so that appropriate measures can be taken.
Crystalline glass defects according to their origin:
- Melt-resistant contaminants of the raw materials and old recycled glass
- Non-melted raw material components
- Corrosion residue of fireproof mineral materials from the smelter
- Devitrification products
Quick and reliable fault diagnosis
Distinction between crystalline inclusions and gas bubbles or processing defects takes place using either automatic inspection and sorting systems or by manual sorting after visual inspection. Segments cut out using a glass cutter or diamond saw can usually be inspected microscopically without further processing. For large, opaque "stones", the defect is ground and diagnostics are carried out in incident light. Smaller inclusions in glass knots often cannot be brought into sharp focus due to the lens effect of the glass bead. Therefore the knot is covered with an immersion solution that has the refractive index of the glass (Figure 1). Furthermore, the microscope configuration presented here also permits quantitative polarized optical measurements; however, this requires flat polished sections of a defined thickness [1, 2, 3].
For nondestructive diagnostics at high magnification of defects located several millimetres under the glass surface, we recommend using the L objectives with extra-large working distance. The 40x Pol objective with coverslip correction is used in addition to the 10x Pol objective for quantitative measurements of thinsection specimens and for conoscopic examinations.
In transmitted light bright field, the shape, colour and relative refractive index of the surrounding glass can be determined by relief of the inclusion. Decentring the condenser or introducing variableopening shutters allows oblique illumination for contrast enhancement. Oblique illumination creates strong relief streaks that are virtually invisible with optimally set Köhler illumination.
In transmitted light polarization contrast, isotropic and anisotropic materials can be differentiated. For idiomorphic crystals, the extinction position can be determined and using the lambda plate, the order of the birefringence can be estimated. Incident light bright field and incident light polarization contrast are suitable only for defects on the glass surface or for ground specimens. Oblique incident illumination combined with transmitted light allows surface details and colours of the inclusions to be identified.
Examples of crystalline inclusions
Tin oxide (SnO2)
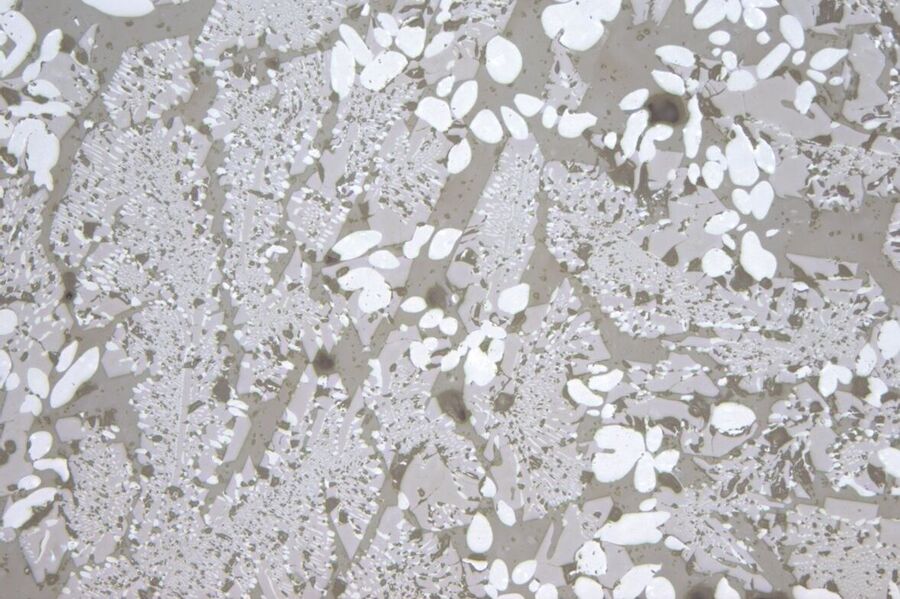
The heating electrodes of some smelters consist of melt-resistant tin oxide. In case of overload, electrode material can flake off, resulting in the typical aggregates of blue, xenomorphic grains (primary tin oxide). At high temperatures, these dissolve after a little while, forming what is known as a knot. At a lower temperature, long prismatic tin oxide crystals (secondary tin oxide) can grow as thin needles (Figure 2) or felt-like aggregates (Figure 3).
Zirconium oxide (ZrO2) and corundum (Al2O3)
Zirconium oxide and corundum are components of the fireproof minerals in smelters (Figure 4). Under normal loads, the resistant zirconium oxide dissolves in a slow, "well-tempered" manner. Large amounts of zirconium oxide glass defects indicate strong local corrosion, such as that due to thermal overload or excessive flow. Zirconium oxide occurs in its original compound as small white inclusions or forms typical dendritic crystals (Figure 5). Al2O3 is more easily dissolved in the glass melter and usually forms glass knots and streaks. However, corundum can also take the form of rounded grains with typical inclusions (Figure 6).
Tridymite/cristobalite (SiO2)
Tridymite, and less frequently, cristobalite, forms as a devitrification product on SiO2-enriched glass, for example when volatile components such as alkalis or boroxide evaporate. Tridymite forms typical crystal aggregates with 60° angles (Figure 7).
References
- Jebsen-Marwedel, H., Brückner, R.: Glastechnische Fabrikationsfehler. 3rd edition, Springer Verlag, Berlin, Heidelberg, New York 1980, VIII + 623 pp.
- Clark-Monks, C., Parker, J. M.: Stones and Cord in Glass. Society of Glass Technology, Sheffield 1980, VIII + 200 pp.
- Begley, E. R.: Guide to Refractory and Glass Reactions. Cahner’s Publishing Co., Boston, Mass. 1970, IX + 149 pp.

![Optical elimination of the lens effect of a glass knot by immersion in a glued-on piece of pipe (graphic to [1], image 3.63) Fig. 1: Optical elimination of the lens effect of a glass knot by immersion in a glued-on piece of pipe (graphic to [1], image 3.63)](/fileadmin/_processed_/2/a/csm_POL-Illu_2_e_pass_61499229dd.jpg)