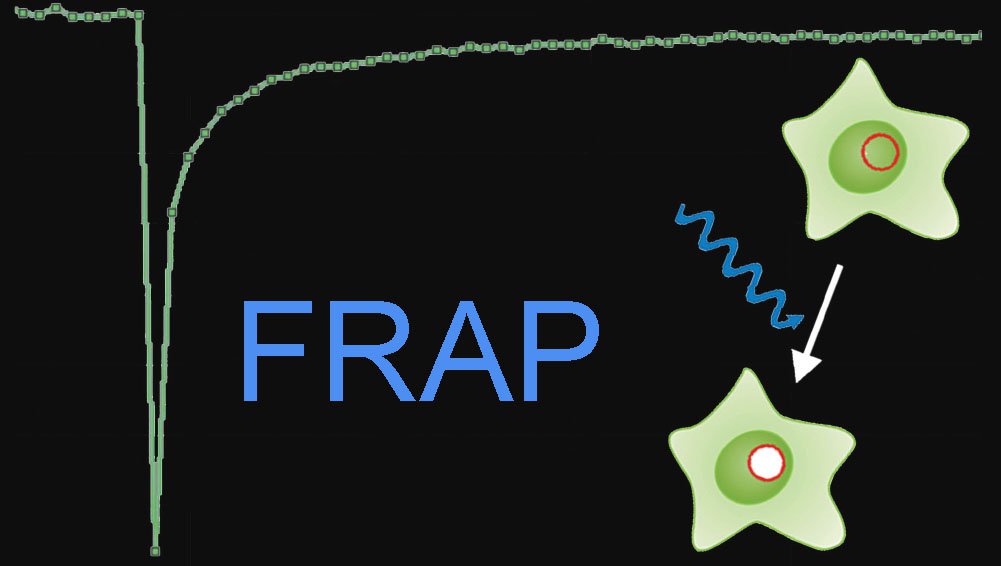
Fluorescence Recovery after Photo Bleaching (FRAP)
The FRAP technique was first used to analyze the mobility of individual lipid molecules within a cell membrane. FRAP can also be used to study protein dynamics outside the membrane: a region of interest within the cytoplasm or cellular structures within the cell can be monitored.
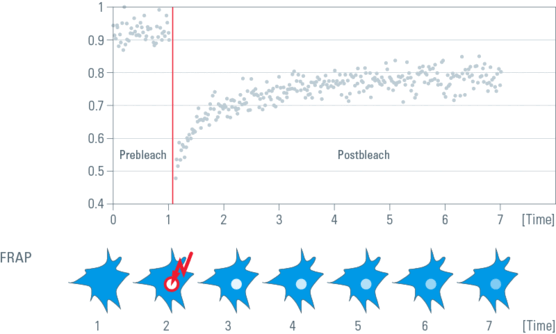
Photobleaching experiments can be conducted with confocal laser scanning microscopes where the laser is used at high intensity for bleaching and low intensity for image recording. It employs irradiation of a fluorophore in a living sample with a short laser to erase fluorescence followed by time-resolved image recording of the sample. In consequence there are three different image sequences: The prebleach sequence with low laser power setting to have a reference value, the bleach sequence with high laser power inside the ROI and the postbleach setting with low laser power setting to examine the recovery of fluorescence. All other bleaching methods are derived from this basic principle (Figure 1).
Fig. 1: Top: Fluorescence intensities of the Bleach-ROI in a time series, the bleach interval is marked red. Bottom: The same cell in a schematic drawing within a time series. At time point two, a region of interest (ROI) within a cell is bleached (arrow). Non bleached molecules are moving into the ROI. The speed of redistribution can be determined.
Typically, the protein of interest is made fluorescent by expression as a GFP (green fluorescent protein cloned from the jellyfish A. victoria) fusion protein or by tagging the protein of interest with reactive ligands which then binds a fluorescent dye. The most common way to fluorescently tag proteins in living cells is GFP technology. Of the different spectral GFP mutants EGFP is the best because of its high quantum yield, its low tendency of photobleaching as well as its relative photostability during post-bleach image acquisition. The speed of redistribution is dependent on molecule size or viscosity of environment or degree of interaction with other molecules or intracellular transport processes. Additionally, the ratio between mobile and immobile molecules can be determined.
As there are numerous possibilities of experiments depending on the application, there is no general recipe for types of experiments. Please refer to the literature cited in the references part.
Fluorescence loss in photo bleaching (FLIP)
Typically, FLIP is used to examine whether cellular organelles like ER or Golgi apparatus are interconnected. A FLIP experiment can gain insight into whether molecules are mobile, immobile or restricted to compartments.
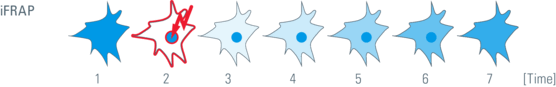
Inverse FRAP
In contrast to FRAP, inverse FRAP (i-FRAP) allows direct analysis of the fluorescent molecules. The fluorescent molecules outside an organelle are bleached. Thus, the efflux of fluorescent molecules out of organelles can be monitored directly without bleaching them. A disadvantage is that this method requires lot of light intensity to bleach the whole cell.
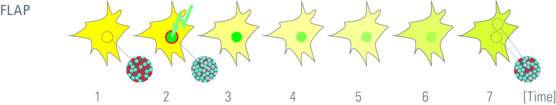
Fluorescence localization after photobleaching
Fluorescence Localization After Photobleaching (FLAP) is a ratiometric method which can be applied to two channels. The experiment requires two different fluorescent labels and only one of the two labels is bleached. The two labels can be tagged to two proteins or one. The non bleached population is the reference measure. The ratio of the bleached and of a second non-bleached area then gives insight into mobility of the proteins. In contrast to FRAP and FLIP, it is a direct measurement method and can be applied to structures which change their morphology rather fast.
Useful links
- Analysis of FRAP Curves, Kota Miura: www.embl.de/eamnet/frap/FRAP6.html
- Introduction to FRAP, Timo Zimmermann: www.embl.de/eamnet/downloads/courses/FRAP2005/tzimmermann_frap.pdf
- Video on FRAP Technique from Essential Cell Biology, 3rd Edition
Alberts, Bray, Hopkin, Johnson, Lewis, Raff, Roberts, & Walter
ISBN: 978-0-8153-4129-1: www.youtube.com/watch?v=LicQb_SnCSI - Lecture of Jennifer Lippincott-Schwartz, Part 2: Photobleaching and Photoactivation: www.youtube.com/watch?v=bhjP9PqfJRE
- Online Manual fot the MBF-ImagsJ Collection: www.macbiophotonics.ca/imagej/intensity_vs_time_ana.htm
References
- Axelrod D, Koppel DE, Schlessinger J, Elson E, Webb WW: Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J 16:9 (1976) 1055–69.
- Phair RD, Misteli T: Kinetic modelling approaches to in vivo imaging. Nat Rev Mol Cell Biol 2:12 (2001) 898–907.
- Snapp E, Altan N, Lippincott-Schwartz J: Measuring protein mobility by photobleaching GFP-chimeras in living cells. Current Protocols, Online Posting Date: August 2003.
- Lippincott-Schwartz J, Altan-Bonnet N, Patterson GH: Photobleaching and photoactivation: following protein dynamics in living cells.
- Phair RD, Misteli T: High mobility of proteins in the mammalian cell nucleus. Nature 404:6778 (2000) 604–9.
- Dundr M, Misteli T: Measuring Dynamics of Nuclear Proteins by Photobleaching. Current Protocols, Online Posting Date: August 2003.
- Dunn GA, Dobbie IM, Monypenny J, Holt MR, Zicha D: Fluorescence localisation after photobleaching (FLAP): a new method for studying protein dynamics in living cells. J Microscopy 205 (2002) 109–112.