Interview with Dr. Shigeki Watanabe at the Leica Workshop, Zurich
Dr. Watanabe, thanks a lot for making time to speak about your research. You are investigating brain functions starting with the very basic functionality. Understanding membrane dynamics is thought to be of paramount importance here – can you explain why that is?
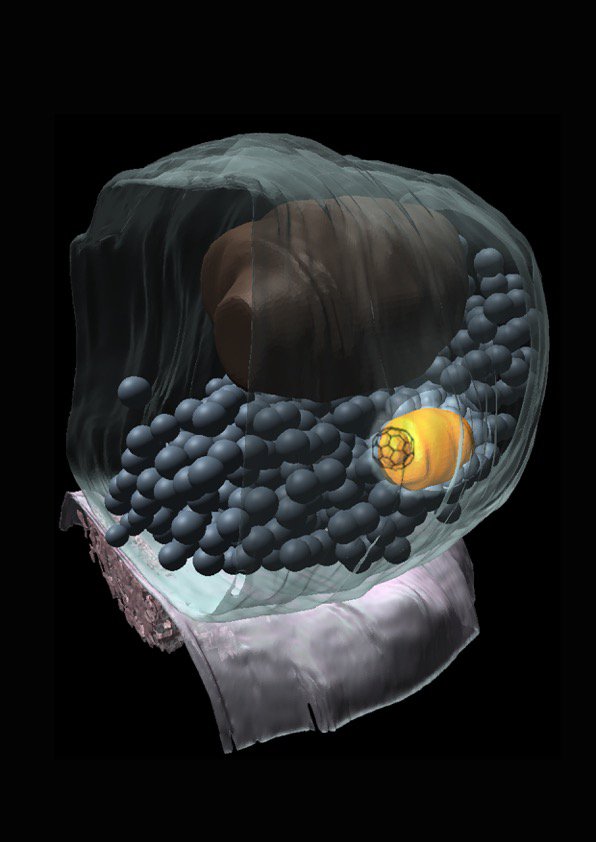
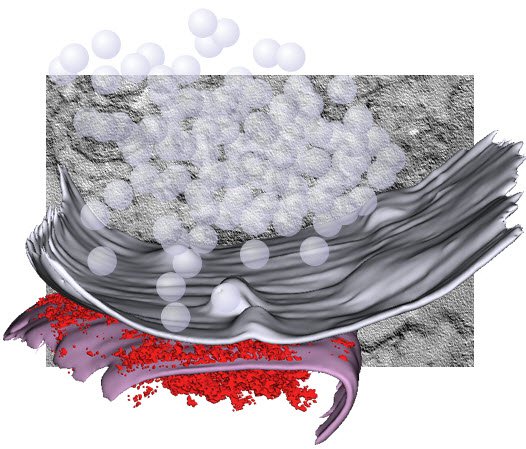
The fundamental unit of the brain is the neuron. Neurons form a network in our brain. The contact site between neurons is called a synapse. To communicate, neurons send out signals at this contact site. Synaptic vesicles, that contain chemical signals or neurotransmitters, fuse with the membrane to release neurotransmitters. Without this process, you won’t have any communication between neurons and therefore you cannot think or you cannot move. So, membrane dynamics at synapses are essential to our life.
It turns out that there are only about 300 vesicles at synaptic terminals but you may be using more than 100 vesicles every second. So in a few seconds, you would deplete all the vesicles from the terminals. Under normal conditions you wouldn’t be able to think or move anymore in a few seconds. But obviously that’s not what happens. Neurons are capable of recycling vesicles at synaptic terminals.
There are many proteins that work at the synaptic terminals to regulate these processes, fusion or regeneration of synaptic vesicles. The defect in these proteins has been implicated in many neurological disorders. Knowing what such proteins normally do and finding out what goes wrong when you have a defect in these proteins, allows us to understand the pathogenesis of these neurological disorders.
Do you already have a deeper understanding of neurological diseases because of the work you have done and the results you have achieved?
Yes, at some levels. We do actually understand the function of a few proteins, for example Synuclein which is associated with Parkinson’s disease. Until recently, it wasn’t known that they regulate the fusion process of the vesicles.
We now have data suggesting that synaptojanin implicated in Down syndrome function at the synaptic terminals to regenerate synaptic vesicles. We begin to understand what the protein does in the cell and we gain understanding of what the mutations or gene duplications do to synaptic functions. However, we are far away from understanding the neurological disorder as a whole.
How would you group neurological disorders with respect to your current findings?
Actually we are more in the basic scientists’ role. We like to understand what synapses normally do. Because if we understand what synapses normally do, we are able to reconstruct what happens if you have a defect in the process. We go from basic understanding to fitting parts of the pathogenesis. All together this is a big leap forward. Those disorders are not going to be one simple thing.
Currently some scientists believe that the agglomeration of metals , e.g. Iron, in the cell leads to neurodegeneration. How would you see those effects coming into play?
It’s a favoured hypothesis that there is a phase transition between the soluble and the polymerized form of the proteins.
If you look at the patient e.g. of Alzheimer’s disease, you find a lot of aggregates all over the place, but even then in the path of pathogenesis the first thing that happens is the dysfunction of the synapses - something happens at the synaptic terminals and changes the property of the synaptic transmission.
...and this is exactly where your research ties in. There are a range methods that are used to research the cells, what techniques do you use?
We apply a lot of cell biological approaches, looking at protein, but the basic assay we do is through electron microscopy. That is what separates us from others in the field. We are pretty unique in using electron microscopy as the primary tool to study synaptic functions.
The reasons for this is that synapses are so small, all the organelles that are within the synaptic terminals are clustered and electron microscopy is the only method that can visualize individual vesicles. Even with the advent of super resolution microscopy you cannot actually delineate every single vesicle in the terminals. Traditional electron microscopy has one big problem: you can only work with fixed samples. But now with the light stimulation we can synchronize the activity of the neurons and follow the membrane dynamics.
So what we do is we express channel rhodopsin in a cell and we stimulate the neurons with light. Then we freeze cells at defined time points after stimulation. This in effect generates flip-books of the membrane dynamics, the technique is called “flash and freeze”. We can use this technique to visualize what the synapses are actually doing.
Would it be appropriate to say "You freeze the thought while it’s happening"?
Yes. So we are freezing the neurons when sending signals. But [laughs] I don’t know if the neurons in the culture have any thoughts.
Would you compare the methodology that you are using to stop-motion live cell imaging omitting the drawback of normal resolution compared to electron microscopy and sampling of “life” with the flash-and-freeze process?
Technically we are not looking at live samples. They are frozen. But we still see the dynamics because of exact knowledge of all time points.
How do you think your research will develop in the future?
We have several lines of research going on in our lab, but mostly we are really interested in understanding the cell biology of the synapses. Obviously one interesting aspect is to study the molecular mechanism of ultrafast endocytosis. This is something we have discovered. Those proteins that I mentioned earlier seem to regulate the process very tightly.
The other aspect we are going into is the receptor trafficking. After the neurotransmitters are released, they are received by the receptors on the post synaptic surface – signals are transmitted. The number of such receptors on the surface of the postsynaptic cell is thought to be the mechanism of learning. The number actually changes depending on the experience. And so we are interested in looking how such changes happen during the synaptic transmission.
Is that in some way related to how learning is achieved by human beings?
That is what people think. The strength of the synaptic connection is what can be modulated based on experience. Some synapses would have more receptors, some synapses would lose receptors. There may also be new synaptic connections. So the neurons in their environment are never sleeping. We would like to understand the molecular mechanisms underlying these modulations.
The last thing is actually kind of funny. People think of the presynaptic terminal as one field, post synaptic receptor trafficking is another field, although they are closely positioned in space. I hope to study synapses as a whole and would like to understand the trans synaptic mechanism - how those synapses are actually connected and then how those synaptic vesicle fusions work. We would really like to understand how this is coordinated in space and time.