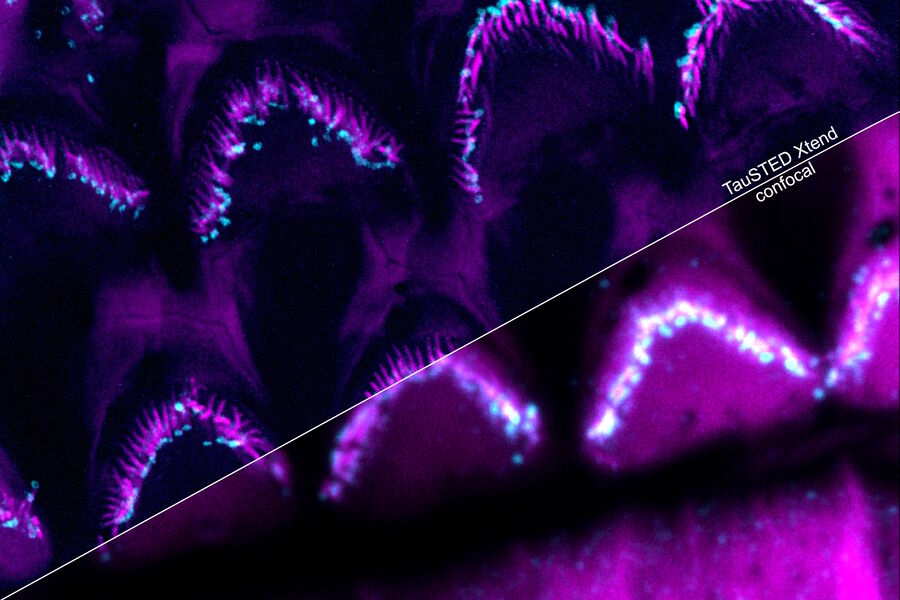
Perform longer live-cell imaging
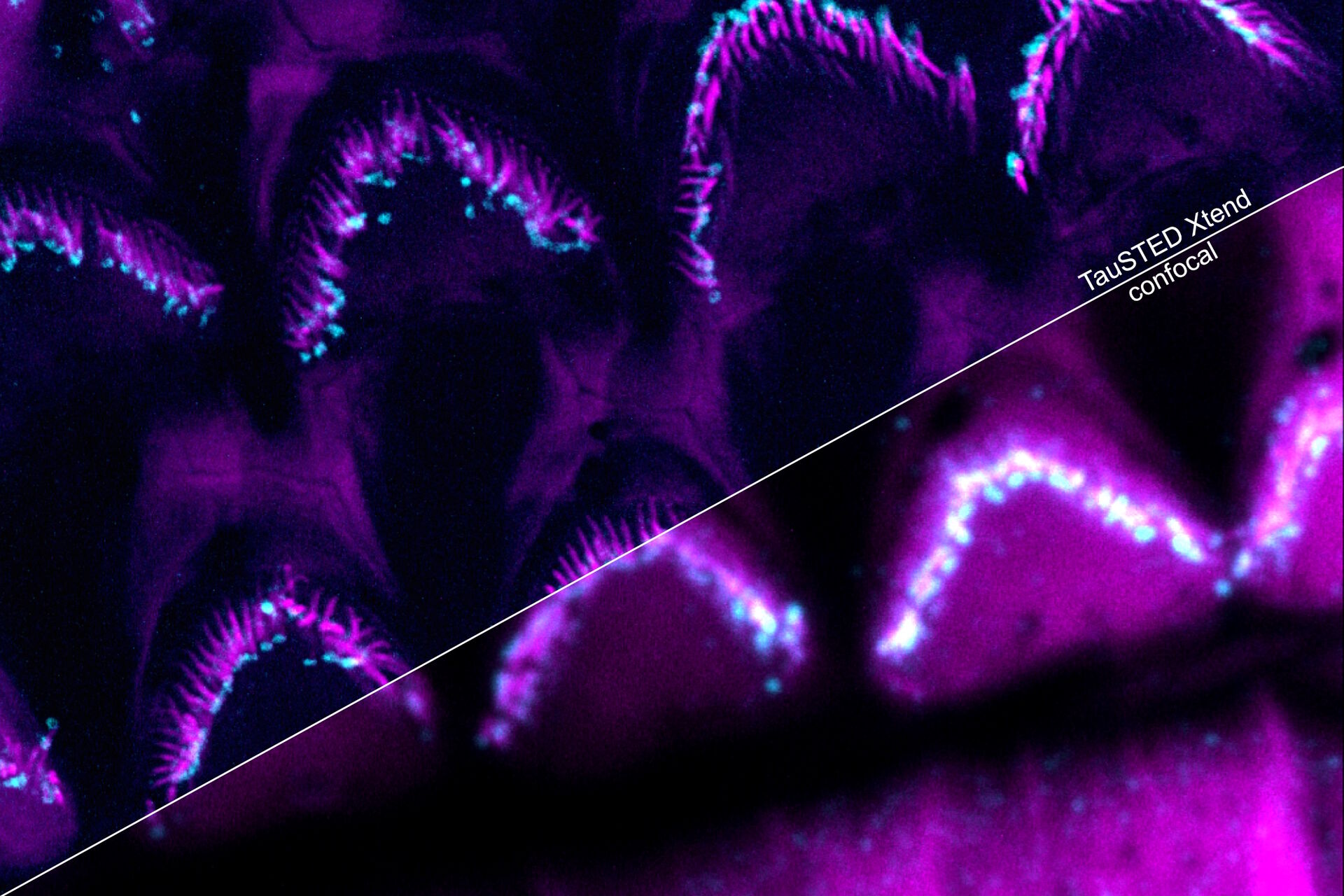
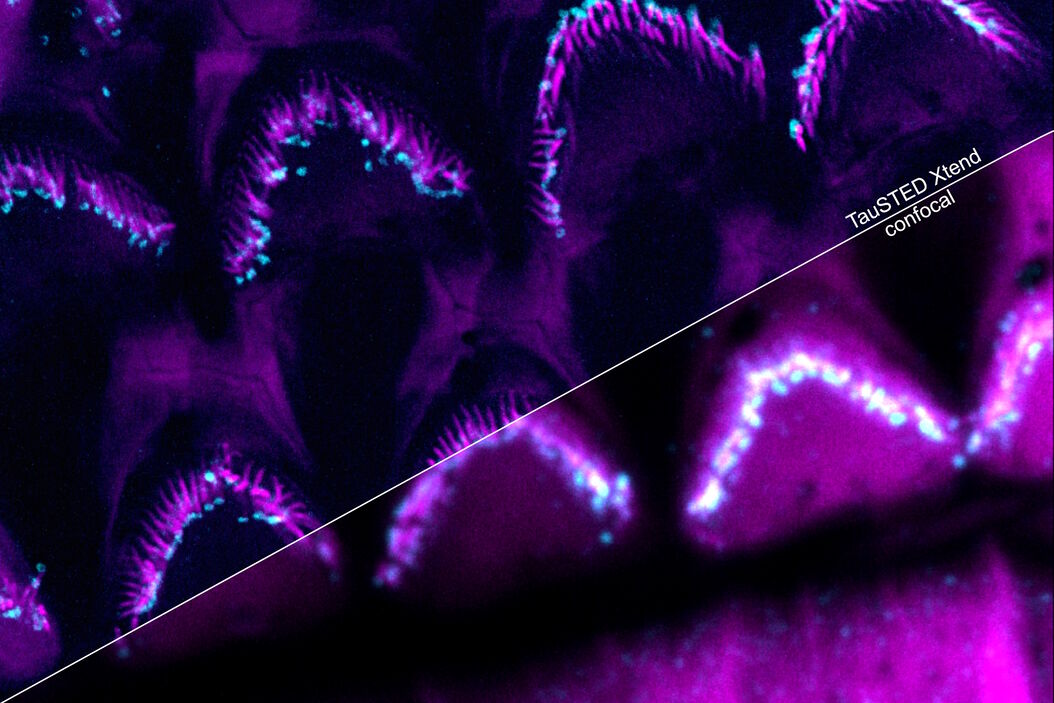
Protect your specimen while performing longer time-lapse experiments with more frames and larger volume imaging at cutting-edge resolution by using lower light doses.
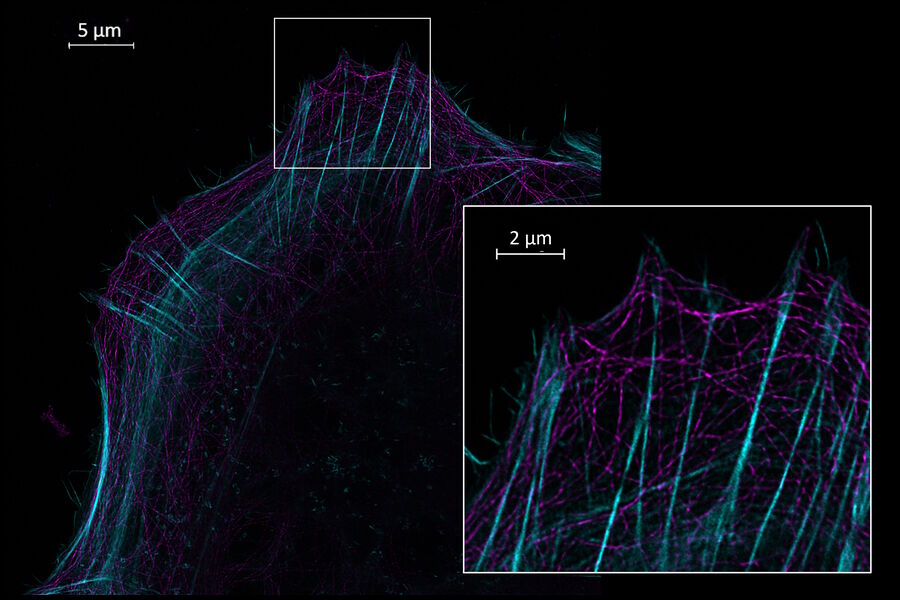
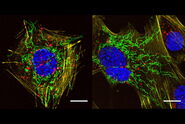
Use your preferred protocols for STED
TauSTED Xtend offers new opportunities for your single-and multicolor experiments using green fluorescent proteins and fluorophores that are a workhorse in life science research and currently underused in studies at the nanoscopic level. With TauSTED Xtend, you can take your experiments effectively down to the nanoscale using protocols you are familiar with.
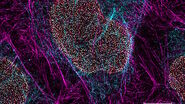
Expanded multicolor imaging
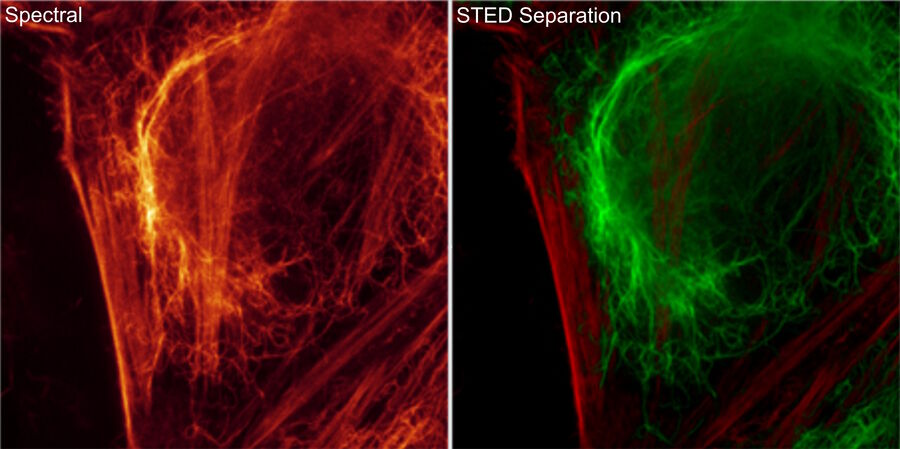
Expand the palette of fluorophores suitable for your investigation by leveraging the unique spectral capabilities of STELLARIS. Moreover, using lifetime-based dye separation with FALCON, additional dyes can be separated. This gives you the flexibility to combine multiple dyes in a way that reinforces your experimental design.
Direct monitoring of fast processes
Make the most of your "window of opportunity" during experiments by seeing what happens as it happens. With TauSTED Xtend, you can follow the results of your experiments on-the-fly. This gives you the confidence that you are observing the right processes and ensures your work is more efficient.