Introduction
Drosophila melanogaster has been a model organism for genetic studies for more than 100 years and continues to be widely used today for developmental biological research in the areas of genetics, physiology, pathogenesis, and evolution [1,2]. It is relatively easy and inexpensive to care for in large numbers. It breeds quickly, lays many eggs, and has a short life cycle of just 2 weeks [1,2]. The fruit fly has only 4 pairs of chromosomes [3], many of which are large, making it easy to locate mutations [4] and deletions [5]. Its entire genome [6] has been sequenced and many mutations are available for use. The homology of cancer and disease related genes found in both the genomes of D. melanogaster and humans is significant [7,8].
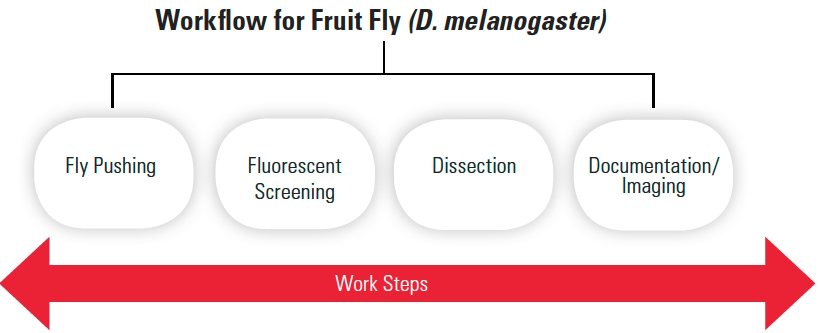
There are several common steps when doing routine work with fruit flies, often D. melanogaster:
- “fly pushing”, i.e., the basic sorting of flies by stereo microscope observation of visible markers, normally done day to day;
- fluorescent screening, similar to fly pushing, but normally involves observing the embryos or larvae with fluorescence stereo microscopy to separate successfully transfected, gene- expressing ones from those which are non-expressing;
- dissection performed via a stereo microscope;
- documentation or imaging, often done with confocal or compound microscopy, but high performance stereo microscopy is also possible.
For clarity, in this text the sequence of steps mentioned above for routine fruit fly work will be referred to as “workflow” (refer to Figure 1). In many cases, the workflow is not a linear “flow” of work steps.
Key considerations for optimizing workflow efficiency with fruit flies
There are several important points which must be considered when attempting to develop a more efficient workflow:
- Identification of the critical tasks for a specific study, for example, sorting fruit flies for selective breeding using visible markers or using fluorescent protein markers;
- Time spent for each step and the overall number of steps: generally the workflow is a daily repetitive, tedious, time-consuming work, which must be done reliably and accurately in order to maintain the required pedigree of the fruit fly;
- Ordinary stereo microscopy: better efficiency with good illumination, resolution, and ergonomic approach;
- Specimen characteristics: in general flies and other insects are largely composed of the polysaccharide chitin and various protein molecules which can contribute to autofluorescence (unwanted background fluorescence signals from the specimen) [9] and decrease the signal-to-noise (S/N) ratio during observation with fluorescence microscopy;
- Fluorescent stereo microscopy: faster workflow with quick switching between filters and between fluorescence and bright field, as well as rapid zooming in and out;
- Dissection: flexible illumination and microscope stand options provide an ergonomic workflow; and
- Documentation/imaging: recording mutant phenotypes, filming behavioral changes, measuring anatomical features, such as wing or leg sizes, and time lapse imaging of cell migration (frequently done with confocal microscopy). Documentation with stereo microscopy often requires different setups of the digital color camera.
Stereo microscope systems for fly pushing and fluorescent screening
Fly Pushing
Fly pushing refers to the daily sorting of flies by anatomical characteristics and phenotype [10]. Flies are generally anesthetized with CO2 and placed on a CO2 perfused pad for sorting. Normally, sorting is done with stereo microscopy.
There are several types of stereo microscopes available from Leica Microsystems which can be used with fruit flies, usually D. melanogaster, for fly pushing, notably the M50, M60, and M80 CMO (common main objective) stereo microscopes [11].
Some of the advantages that these microscopes offer:
- Optics with extremely high light transmission, enabling many details to be seen at a glance with no need to use extra bright illumination that can potentially create shadows or bright spots;
- Eyepieces with a field number (FN) of 23 mm, which can provide a larger field of view (FOV)/object field (OF), giving the user a quick overview of the flies over a large area of the pad for fast, efficient fly pushing (N.B.: the M80 has a smaller FOV than the M50 and M60 – refer to Figure 2);
- The M80 achieves better resolution (maximum numerical aperture [NA] of 0.206) than the M60 and M50 (maximum NA of 0.15) – higher resolution facilitates the identification of subtle phenotypes, such as bristle morphology;
- For the wider FOV of the M50 and M60, depending on the application, plan achromatic objective lenses are normally recommended, while achromatic ones are sufficient for the M80 [12];
- The M50, M60, and M80 can be equipped with the LED2000 or LED2500 all-in-one microscope stand and illumination system which keeps the table top working space free of cables and external light sources (N.B.: normally fruit flies are kept in hundreds of plastic vials and these fill up the working space, occasionally cables from equipment can get bumped by users and accidentally knock some vials onto the ground);
- Ergonomic accessories for stereo microscopes, such as ErgoWedges, ease the strain of repetitive work (N.B.: when microscope users in the laboratory feel more comfortable while fly pushing all day long, they will be more productive, and ergonomic accessories can be retrofit economically to older model Leica microscopes);
- Compact LED light sources, which are attached to the stand, allow the stereo microscopes to be easily moved for cleaning and give the users more free bench space.
The photos below show a typical Drosophila lab with stereo microscopes for fly pushing (courtesy of T. Hummel, Dept. of Neurobiology, University of Vienna, Austria).
Useful information (hints and tips)
Size of microscope base plate
Microscope footprint is a key consideration for every laboratory. Fly pads for CO2 anesthetization are available in different sizes, but all tend to fall off the microscope base during fly pushing, as the base can be too small.
Solutions to this potential problem are:
- Using medium size bases; or
- Alternatively one can use small swingarm (boom) stands and work directly on the bench (refer to Figure 5). The LED3000 spotlight illumination (SLI) with goosenecks can be mounted directly on the swingarm stand and all lighting controls are directly accessible on the goosenecks, so they do not crowd the bench.
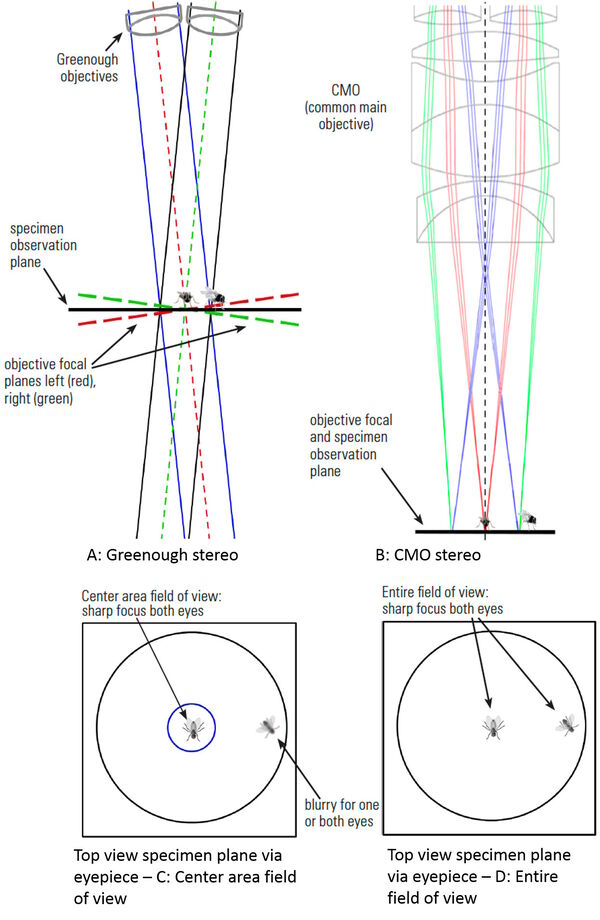
Advantages versus disadvantages of Greenough and CMO (Common Main Objective) stereo microscopes for fly pushing
Greenough advantages:
- Normally longer working distances than CMO stereo microscopes; and
- An economic solution.
Greenough disadvantages:
- Limited modularity; and
- Optical axes of the lens systems (for each eye) converges to a center point where the specimen is placed. The two separate focal planes for each eye does not allow clear focus for both eyes on a specimen positioned away from the center (refer to Figure 6).
CMO advantages:
All specimens are in the same focal plane for both eyes, which leads to a larger useable field of view at a specific magnification value compared to Greenough stereo microscopes [13,14] and allows:
- larger overview with less need to refocus and move the fly pad around;
- faster identification of fruit flies; and
- smoother workflow, making fly pushing less tedious.
Figure 6 compares the optics of a Greenough and a CMO stereo microscope.
Fluorescent screening
In many laboratories, special strains of fruit flies, which have modified genotypes [15] to express markers such as Green (GFP), Red (RFP), Cyan (CFP), and Yellow (YFP) Fluorescent Protein, are used [16]. Typically, the fly larvae or embryos are observed and analyzed with fluorescence stereo microscopy and then selected based on the presence or absence of fluorescent markers, hopefully in the expected organ. Using fluorescent protein markers, like GFP, make the job of sorting non-mutated from mutated larvae much easier. However, in order to gather sufficient data for analysis, many fruit fly larvae must be screened and analyzed.
There are several types of stereo microscopes available from Leica Microsystems which can be used for fluorescent screening of fruit flies: the M165 FC, M205 FA, and MZ10 F (see example images in Figure 7).
Some of the advantages that each microscope can offer users are the following:
Fluorescence stereo microscopes offer fly screeners:
- Best signal-to-noise (S/N) ratio for fluorescence microscopy due to the unique triple beam design;
- The M205 FA has the best resolution (maximum NA of 0.349), followed by the M165 FC (maximum NA of 0.3), and then the MZ10 F (maximum NA of 0.25);
- The M165 FC and the M205 FA use plan apochromatic objective lenses, while achromatic ones are typical for the MZ10 F;
- The M165 FC and the M205 FA have encoded optics for reliable measurements of fly anatomy, e.g., dimensions and surface area of legs, wings, eyes, etc.;
- The M205 FA has the FusionOptics technology [17] for a visual 3D perception through the eyepieces with the highest resolution and depth of field possible, simultaneously and even at high magnification, which is very practical for fluorescent screening;
- The M205 FA is a fully automated fluorescence stereo microscope and, as a result, also can be used for complex imaging like z-stacking and multi-channel acquisition; and
- Ergonomic accessories, such as ErgoTubes, ease the strain of repetitive work by allowing the height and angle of the eyepieces to be adjusted for the comfort of the individual user, leading to higher productivity.
Dissection
Dissection of fruit flies and their larvae is done to allow more detailed studies of the organs and tissues [18]. Generally, a high performance stereo microscope is used for dissection.
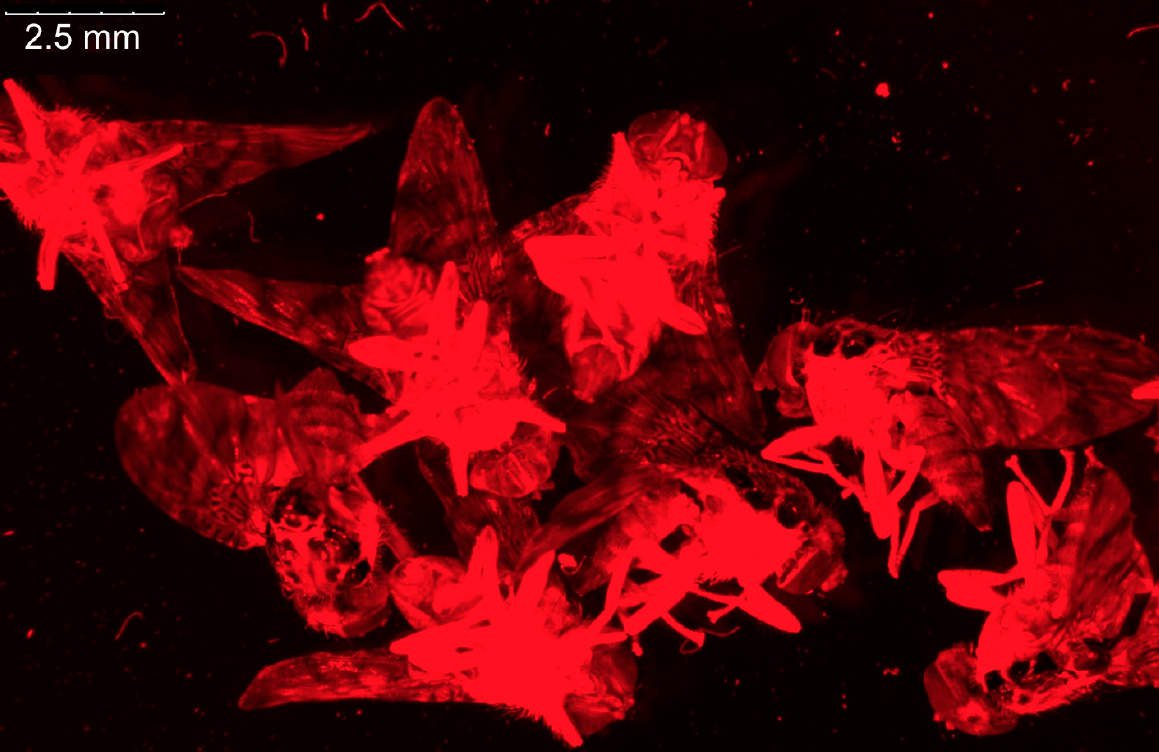
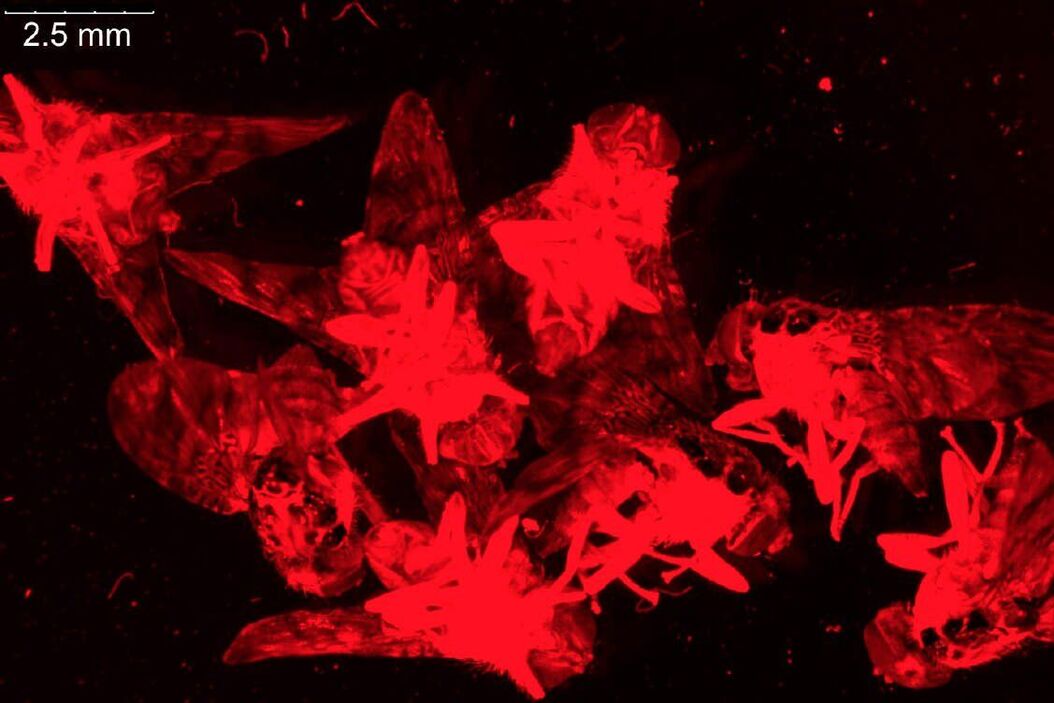
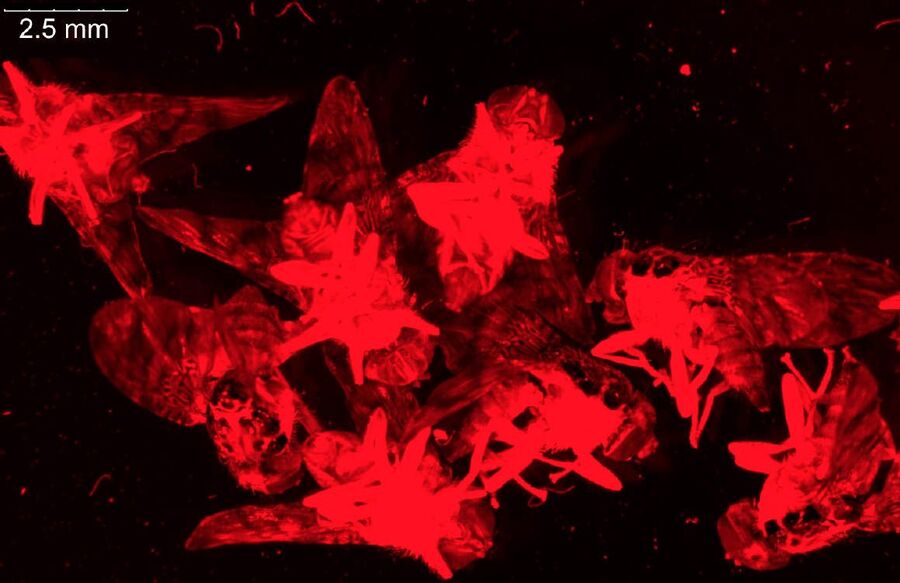
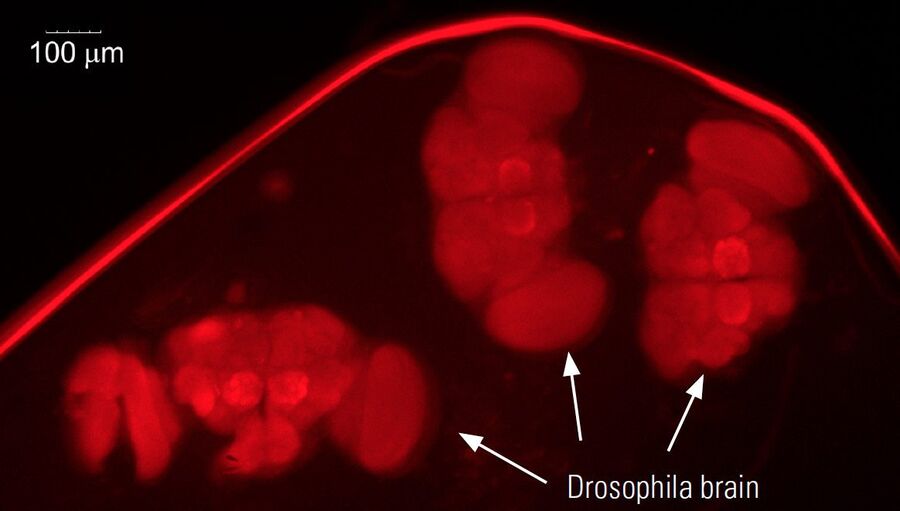
For dissection of fruit flies and larvae, the M80 stereo microscope is often sufficient, but the M125 has higher magnification and resolution and a good field of view which makes it more practical for preparation of fly organs, e.g., brains (refer to the example in Figure 8). Normally, the organs are later imaged using higher resolution confocal, compound, or stereo microscopy.
Documentation/Imaging
Phenotypic characterization of mutations in the eyes, legs, body, and wings of the fruit fly often are documented using encoded stereo microscopy, normally a higher performance system, such as the M205 FA, M165 FC, M205 A, M205 C, M165 FC, or M165 C. To take videos for the observation of fruit fly behavior, a digital microscope can be useful, allowing the capture of high frame rates and large overviews on a relatively small setup. In addition, the Leica Application Suite X (LAS X) software offers features like extended depth of field (EDOF) or image z-stacking for quick, routine fly characterization in 3D. Using encoded Leica stereo microscopes in combination with LAS X software, all images will be correctly calibrated and, thus, guarantee accurate measurements of wing size, leg length, eye surface, and other relevant changes in phenotype.
For many studies, characterization of the fruit fly phenotype must be performed at the subcellular level [19]. Documentation of dissected fruit fly parts to acquire details at the subcellular level is normally done with a confocal or compound microscope.
The M205 FA is fully encoded and automated to allow complex imaging tasks to be done more easily. The encoding and automation provide reliable measurements at the cellular and subcellular level. It uses FusionOptics technology [17] and ergonomic accessories are available.
Conclusion
The fruit fly, Drosophila melanogaster, has been a model organism for developmental biology research for more than a century [1,2]. Its genome is fully sequenced and the disease related genes show appreciable homology to those of humans [7].
The routine workflow for fruit flies, usually D. melanogaster, involves multiple steps using stereo microscopy:
- fly pushing, daily basic sorting of flies;
- fluorescent screening, observing the embryos or larvae; and
- dissection for later documentation or imaging.
Documentation or imaging of the anatomy or whole organs of fruit flies is usually done with stereo microscopy and parts dissected from them with confocal or compound microscopy to obtain subcellular details.
This report refers to examples of scientists and technicians working with fruit flies, such as D. melanogaster, and shows different possible setups with a large range of microscopes and accessories. Because the demands of each laboratory can vary widely, a large range of configurations and instruments are available to address specific tasks in the workflow or even enable more work steps to be performed by one instrument. This short report presents recommended workflows based upon the experiences of different well-established fruit fly labs and can be a very useful reference or guidelines when setting up or expanding a fly lab.
Acknowledgements
We would like to thank Laura Geid and Prof. Thomas Hummel, Dept. of Neurobiology, University of Vienna in Austria for supplying photos of his Drosophila laboratory, as well as, Prof. Bruno Reversade, IMB, A*STAR, Singapore.
References
- Manning G: A quick and simple introduction to Drosophila melanogaster. Drosophila Virtual Library.
- Morgan TH: Biographical – The Nobel Prize in Physiology or Medicine 1933. The Nobel Foundation, Stockholm, Sweden.
- Chromosomes. National Human Genome Research Institute (NHGRI), National Institute of Health (NIH), Bethesda, Maryland, USA.
- Mutation – Talking Glossary of Genetic Terms. National Human Genome Research Institute (NHGRI), National Institute of Health (NIH), Bethesda, Maryland, USA.
- Deletion – Talking Glossary of Genetic Terms. National Human Genome Research Institute (NHGRI), National Institute of Health (NIH), Bethesda, Maryland, USA.
- Genome – Talking Glossary of Genetic Terms. National Human Genome Research Institute (NHGRI), National Institute of Health (NIH), Bethesda, Maryland, USA.
- Reiter LT, Potocki L, Chien S, Gribskov M, and Bier E: A Systematic Analysis of Human Disease-Associated Gene Sequences In Drosophila melanogaster. Genome Res. 11 (6): 1114–25 (2001); doi: 10.1101/gr.169101.
- Herrero J: How to get all the orthologous genes between two species. Ensembl Project, Cambridge, UK.
- Hoff F: How to Prepare Your Specimen for Immunofluorescence Microscopy. Science Lab.
- Phenotype – Talking Glossary of Genetic Terms. National Human Genome Research Institute (NHGRI), National Institute of Health (NIH), Bethesda, Maryland, USA.
- Goeggel D: The History of Stereo Microscopy – Part III: The 19th Century – Breakthrough of Modern Microscope Manufacturing. Science Lab.
- Objective Classes. Leica Microsystems.
- Rottermann R, and Bauer P: How Sharp Images Are Formed: Depth of Field in Microscopy. Science Lab.
- Goeggel D: Factors to Consider When Selecting a Stereo Microscope. Science Lab.
- Genotype – Talking Glossary of Genetic Terms. National Human Genome Research Institute (NHGRI), National Institute of Health (NIH), Bethesda, Maryland, USA.
- Greb C: Fluorescent Proteins – Introduction and Photo Spectral Characteristics. Science Lab.
- Goeggel D, Schué A, Kiper D, Müller C, What is the FusionOptics Technology: Leica stereo microscopes with FusionOptics combine high resolution and depth of field for ideal 3D perception of samples, Science Lab (2023) Leica Microsystems.
- Maimon I, and Gilboa L: Dissection and Staining of Drosophila Larval Ovaries. Jove, doi: 10.3791/2537.
- De Bock J: Mosaic Images: Bringing Together What Belongs Together. Science Lab.