Medical Device Quality Assurance and Quality Control
Reliable image capture and analysis for your regulatory affairs (RA) and QA/QC documentation: With microscope solutions from Leica Microsystems, you can rely on the results of visual inspection and documentation for medical devices.
Leica Microsystems offers complete solutions for visual inspection which deliver reliable image data. Identify, analyze, validate, and document defects efficiently as a means to achieve compliance with regulations concerning your medical devices. Especially, when it comes to higher risk class II and III medical devices, such as stents, dental implants, catheters, and many other implantable ones, you can benefit from these solutions.
Please contact us for personal expert advice on our microscopy solutions for medical device quality assurance and quality control.
Select your field of application
Challenges for medical device QA/QC
Quality assurance and control (QA/QC) of your medical devices can require a lot of visual inspection with a microscope. You can encounter the following challenges:
- Difficulty to maintain consistent, efficient inspection, evaluation, and reporting of results across multiple operators due to the changing of ever-stricter regulations
- High effort needed to achieve reliable traceability of documentation required for highly regulated environments
- Paper-based documentation of visual inspection is time-consuming and effort intensive, because there are many steps, such as document hand-overs and physical storage and eventual digitization of documentation
- Spending many hours at a microscope can lead to strain due to an uncomfortable posture
These challenges can be overcome with digitally enhanced solutions and ergonomic accessories for microscopes offered by Leica Microsystems.
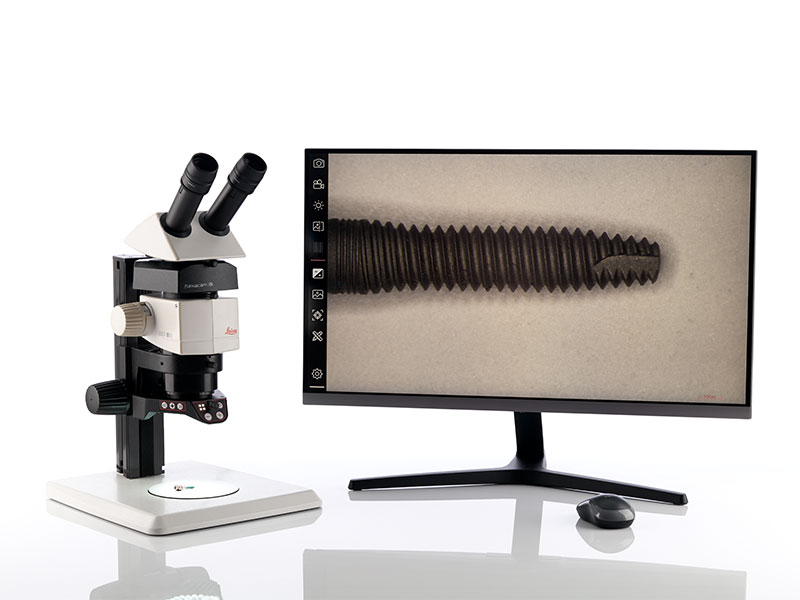
Perform reliable and efficient inspection
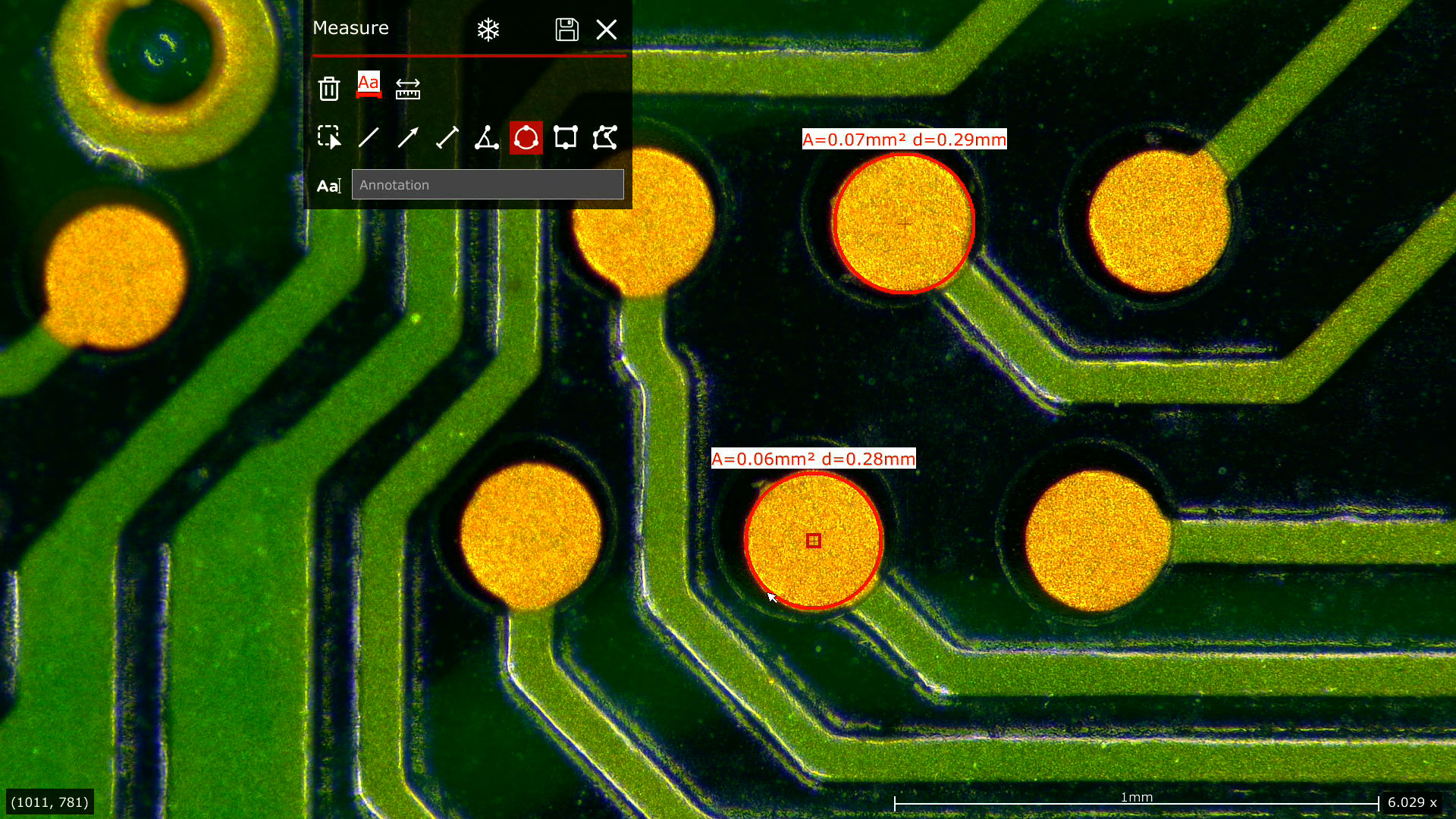
For medical device manufacturing, consistent inspection of defects, such as burrs, chips, and scratches is a necessity to meet the quality requirements. For compliance with standard procedures across multiple users, ensuring consistency is crucial. Additionally, it is important to rapidly adapt and implement changes to procedures.
- Leica stereo microscopes use high-quality optics and digital cameras enabling you visualize components in 2D and 3D with optimal contrast and resolution. They also provide distortion-free, sharp images and allow you go quickly from overview to details
- A more efficient inspection is possible with the FusionOptics technology which provides images with larger depth of field and maximum resolution compared to standard stereo microscopes

Measurements of lengths, areas, angles, etc. have been made using the Leica LAS X software.
Efficient RA/QA documentation
For medical devices, a proficient digital documentation workflow can also help you adapt and meet more easily ever-stricter RA/QA requirements.
Leica digitally enhanced inspection solutions allow you obtain reliable, consistent results, even across multiple users, giving you peace of mind during documentation.
Advantages for RA/QA documentation with Leica medical device solutions:
- Easy digital report generation with fewer manual steps and automated data transfer into an immediately available centralized database
- No need for physical storage and digitization of paper documentation
- Fully electronic approval of documentation for reliable traceability
- By adding the Exalta smart device for traceable microscopy to your microscope solution, you can achieve reliable and efficient reporting based on a smooth digital workflow.
Increase comfort with ergonomics
Performing visual inspection can be intensive because of physical discomfort or strain causing users to lose concentration and potentially make errors. Help users to work comfortably even if they spend the whole day working with a microscope.
- By reducing strain with ergonomically designed microscope workstations, user health is promoted and this improves concentration leading to better quality of work
- Physical comfort of the user also means fewer absences and work-related health issues
- If microscopes are used by multiple users across shifts, then ergonomic accessories enable adaptation for the well-being of any individual
Leica Microsystems offers a variety of Ergo accessories for the M series stereo microscopes. You can improve performance with a well-designed work environment which is adaptable for different users.

Find the solution for your needs
Two dedicated solutions are available for your specific inspection tasks to help you ensure the quality of your medical devices.
- Standard: Ideal when operators work less than 2 hours continuously with the microscope and only occasionally performs QC work.
- Ergo: Ideal when operators work more than 2 hours continuously with the microscope and intense concentration on the QC work over long hours is crucial.

Standard Configuration
- Ivesta 3 Greenough stereo microscope with documentation port
- Flexacam c5 microscope camera
- Optional: Mouse and keyboard

Ergo Configuration
- M60 stereo microscope
- Flexacam c5 microscope camera
- Optional: Ergonomic accessories, mouse and keyboard