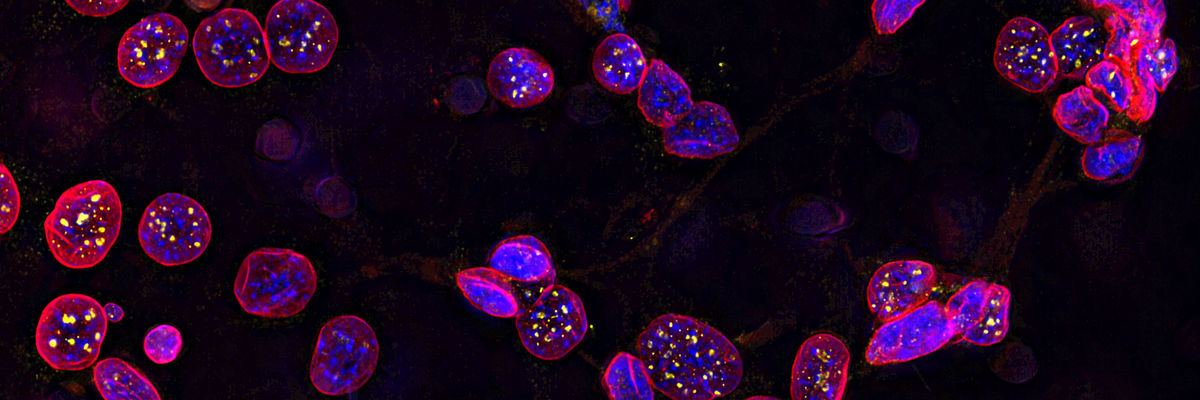
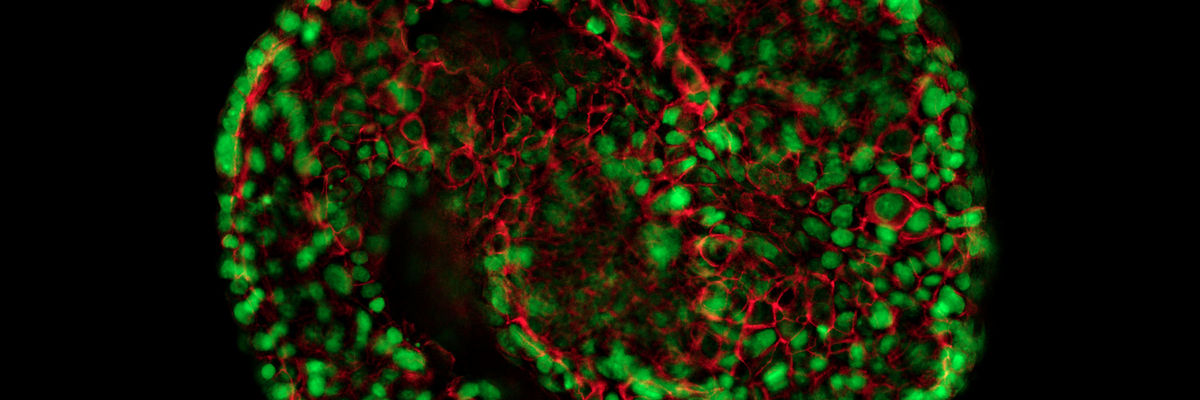
Expand Your Research
With our modular concept, you can tailor your confocal microscope to your current needs and upgrade with additional functionalities at any time
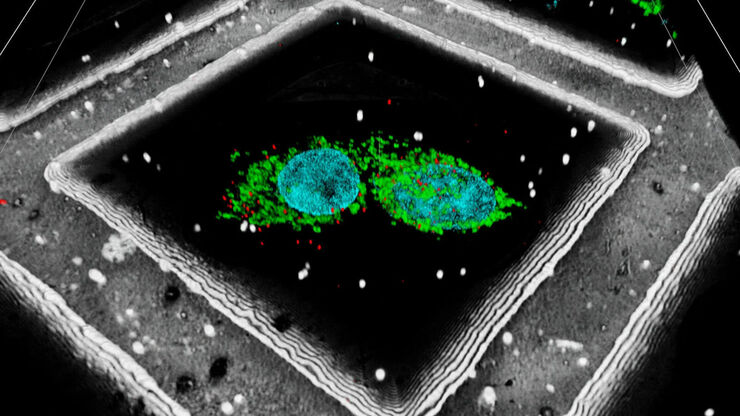
STED – Optical Super-Resolution
STED (STimulated Emission Depletion) delivers powerful and cuttingedge multicolor, deep, and live-cell nanoscopy, for 2D and 3D applications. STED enables you to characterize details in your sample and unveil molecular relationships at the nanoscale level.
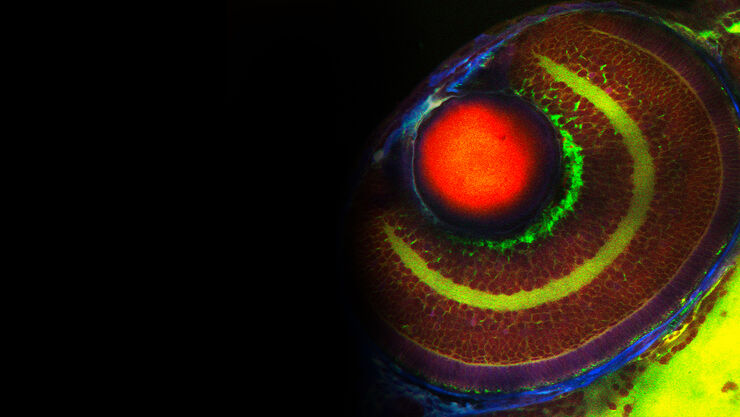
DIVE – Deep In Vivo Explorer
DIVE (Deep In Vivo Explorer) is the first multiphoton microscope with spectrally tunable detection. Equipped with 4Tune, a tunable, nondescanned detection unit, DIVE offers you unlimited flexibility and enables you to develop new multicolor deep in vivo experiments.
FALCON – FAst Lifetime CONtrast
Lifetime imaging in an instant. STELLARIS 8 FALCON (FAst Lifetime CONtrast) is the future of functional imaging. Harness the power of fluorescence lifetime to investigate cellular physiology and explore dynamics in living cells.
DLS – Digital LightSheet
With DLS (Digital LightSheet) you can benefit from two imaging systems in one: a full confocal and an easy-to-use light-sheet microscope with single plane illumination, making your research more versatile.
Cryo – Cryo-Electron Tomography
STELLARIS Cryo is a confocal microscope system that helps you to target your area of interest for cryo-electron tomography (CryoET). STELLARIS Cryo gives you the precision to target reliably, while offering superior performance you can count on and sample safety for your experiments.
CRS – Label Free Imaging
The CRS (Coherent Raman Scattering) technology exploits image contrast< arising from characteristic vibrational states of different molecules within a specimen. As no labeling is required, the specimen remains almost unaffected from preparation and imaging.
Find the right STELLARIS platform for your research needs!
Answer the short questionnaire to find the right solution for your requirements. If you prefer to contact a Leica representative, please fill out the contact form.
Contact us{{ question.questionText }}
Please select an answer!
Best Match
{{ resultProduct.header }}
{{ resultProduct.subheader }}
{{ resultProduct.description }}
{{ resultProduct.features }}
Request Your Information Package
What is a confocal microscope?
Confocal laser scanning microscopy (CLSM) images samples by means of optical sectioning. The benefit of confocal imaging is a dramatically increased contrast by the removal of out-of-focus fluorescence signals. It is well suited for time-lapse imaging, fluorescence lifetime imaging microscopy (FLIM), fluorescence recovery after photobleaching (FRAP), Förster resonance energy transfer (FRET), and fluorescence correlation spectroscopy (FCS) measurements.
What are confocal microscopes used for?
Confocal microscopes are often used for the visualization and analysis of subcellular structures and biomolecules, such as proteins, in fixed and live specimens. You can find out more about applications where confocal microscopy is used from Science Lab, the microscopy knowledge portal of Leica Microsystems.
When was the confocal microscope invented?
In 1957 Marvin Minsky applied for a patent which contained for the first time a description of a working confocal microscope, but it was largely ignored by the scientific community. The method was improved extensively over the years with advances in laser technology and detectors. Confocal microscopy became a generally accepted and popular technique by the 1980s.
Confocal Microscopes
Confocal microscopes from Leica Microsystems are partners in top level biomedical research and surface analysis in material science applications, offering unprecedented precision in three-dimensional imaging and exact examination of subcellular structures and dynamic processes.
About Confocal
Confocal Laser Scanning Microscopy (CLSM) is one of a series of methods to generate slices from microscopic samples by means of optics. The sample stays intact, and the slicing may be repeated many times. True Confocal Scanning (TCS) is a technique, where only a single, diffraction limited spot is illuminated and observed at a time. The benefit of confocal imaging is a dramatically increased contrast by removal of out-of-focus haze. Z-sequences of optical slices (3D image stacks) are sources for subsequent rendering as anaglyphes, depth-coded maps or 3D movies. TCS is also very well compatible with multi-fluorescence imaging, time-lapse imaging, FLIM, FRAP and FCS measurements – plus a whole world of spectral applications.
Frequently Asked Questions Confocal Microscopes
In preparation for cryo-confocal microscopy samples are rapidly frozen to low temperatures (typically below -150°C) using liquid nitrogen or other cryogens. Cryogenic freezing helps protect structural integrity of the samples and prevents ice crystal formation. The cryo environment stabilizes samples preserving native structures, reduces photobleaching and photodamage, and enables imaging of hydrated samples without dehydration or fixation. Cryo-confocal microscopy can be combined with cryo-electron microscopy (Cryo-EM) for correlative studies (CLEM). Cryo CLEM enables detailed visualization and analysis of samples in a near-native state, providing valuable insights into the organization and ultrastructure of proteins and macromolecules.
STED super-resolution microscopy allows imaging at resolutions well below the diffraction limit based purely on physical principles. The key concept of STED relies on the ability to control the states of a fluorophore, i.e., emitting vs. dark states. The emission of fluorophores within a diffraction-limited spot is confined to a sub-diffraction region by overlaying a donut-shaped laser beam of appropriate wavelength (STED beam) onto the excitation beam of a confocal microscope. This approach forces the fluorophores under the effect of the STED beam to return to the ground state before spontaneously emitting a photon. The effective focal volume can be reduced up to a few tens of nm. For more information, refer to: Nanoscopy Meets Lifetime - Introducing the new TauSTED
Fluorescence lifetime is a measure of how long a fluorophore remains in its excited state before returning to the ground state by emitting a photon. The characteristic time of this emission measured on a population of fluorophores is called fluorescence lifetime, which is in the range of picoseconds to nanoseconds. Fluorescence lifetime is a characteristic parameter of a given fluorophore that may change with its local environment or conformational state, while remains independent of the fluorophore concentration. Local environmental factors are ion concentration, pH, lipophilicity, or the presence of other molecules close to the fluorophore. This fact makes FLIM ideal for functional imaging which enables investigation of molecular function and interactions. Additionally, FLIM can be useful for distinguishing fluorophores with overlapping emission spectra or eliminating unwanted background fluorescence. For more information on FLIM, refer to: Fluorescence Lifetime Imaging
When imaging deep inside thick specimens and samples, performing confocal fluorescence microscopy with one-photon excitation can be challenging due to the scattering of visible light. The maximum imaging depth achievable for one-photon excitation is around 100 µm. In contrast, multiphoton microscopy takes advantage of multiphoton excitation with infrared light which has reduced scattering due to the longer wavelengths. This fact makes multiphoton microscopy ideal for deep tissue imaging of thick specimens and samples. Multiphoton microscopy has been used for such things as visualizing the complex architecture of the whole brain as well as the study of tumor development, metastasis, and immune response in organisms. For more details, refer to the tutorial: Principles of Multiphoton Microscopy for Deep Tissue Imaging
Light-sheet microscopy, also referred to as selective-plane illumination microscopy, is a gentle way of imaging sensitive samples or fast biological processes in live specimens. The optical scanning is only done in a single plane and detected from the perpendicular direction. The excitation is done with a thin light-sheet that illuminates the focal plane and spares the sample from out-of-focus excitation. DLS microscopy uses a digital camera and is advantageous for fast imaging of live specimens. For more details, refer to the article: Confocal and Digital Light Sheet Imaging
A microscopy method which takes advantage of the intrinsic vibrational contrast of molecules within biological specimens. It is normally done in 2 ways: either with coherent anti-Stokes Raman scattering (CARS) or stimulated Raman scattering (SRS). The big advantage of CRS is that no labeling of the specimen is required, because the image contrast arises from the spectroscopic properties of various molecular species present in the specimen. As there is no labeling, CRS can allow specimens to be imaged in a nearly pristine state. For more information, refer to: Coherent Raman Scattering (CRS) Microscopy
It is an optimal light source for confocal microscopes. The white light laser (WLL) consists of a high-energy pulsed IR-fiber laser that is fed through a photonic crystal fiber to generate a spectral continuum. Small bandlets are selected from that continuum by means of an acousto-optical beam splitter (AOBS). The WLL provides excitation tunable from blue to red throughout the spectrum. For more details, refer to the article: STELLARIS White Light Laser
For fluorescence microscopy, it is desirable to filter and separate specific color bands for the excitation and emission of fluorophores. In the past, filter and beam splitting was usually conducted with planar optical elements like filters and mirrors. They have the limitation of fixed specification and slow exchange. An entirely different approach is the employment of acousto-optical elements for both excitation control, done with an AOTF, and excitation emission separation, done with an AOBS. These acousto-optical devices allow flexible tunability and high-speed switching. For more details, refer to the article: Acousto Optics in True Confocal Spectral Microscope Systems
Latest News
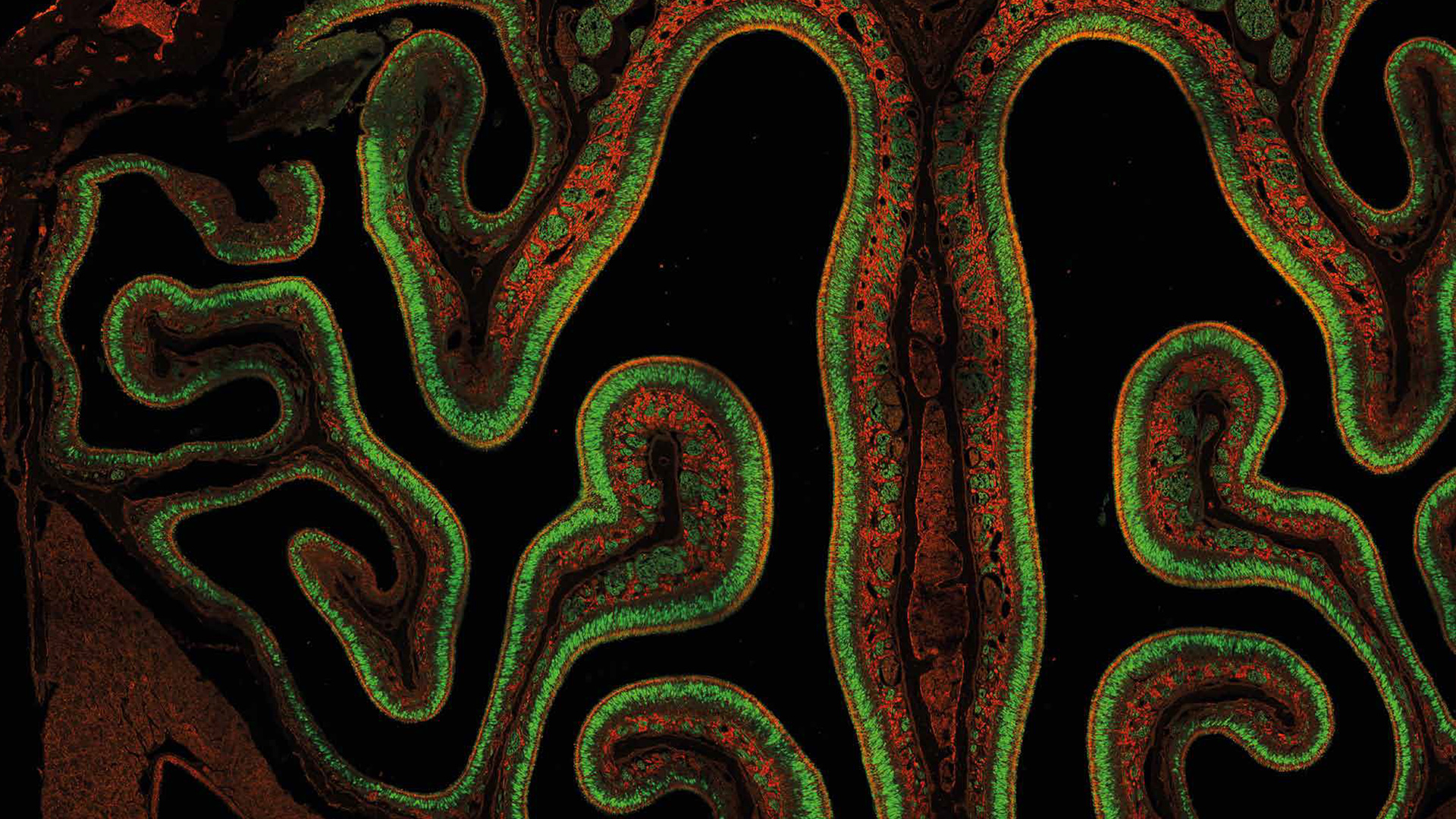
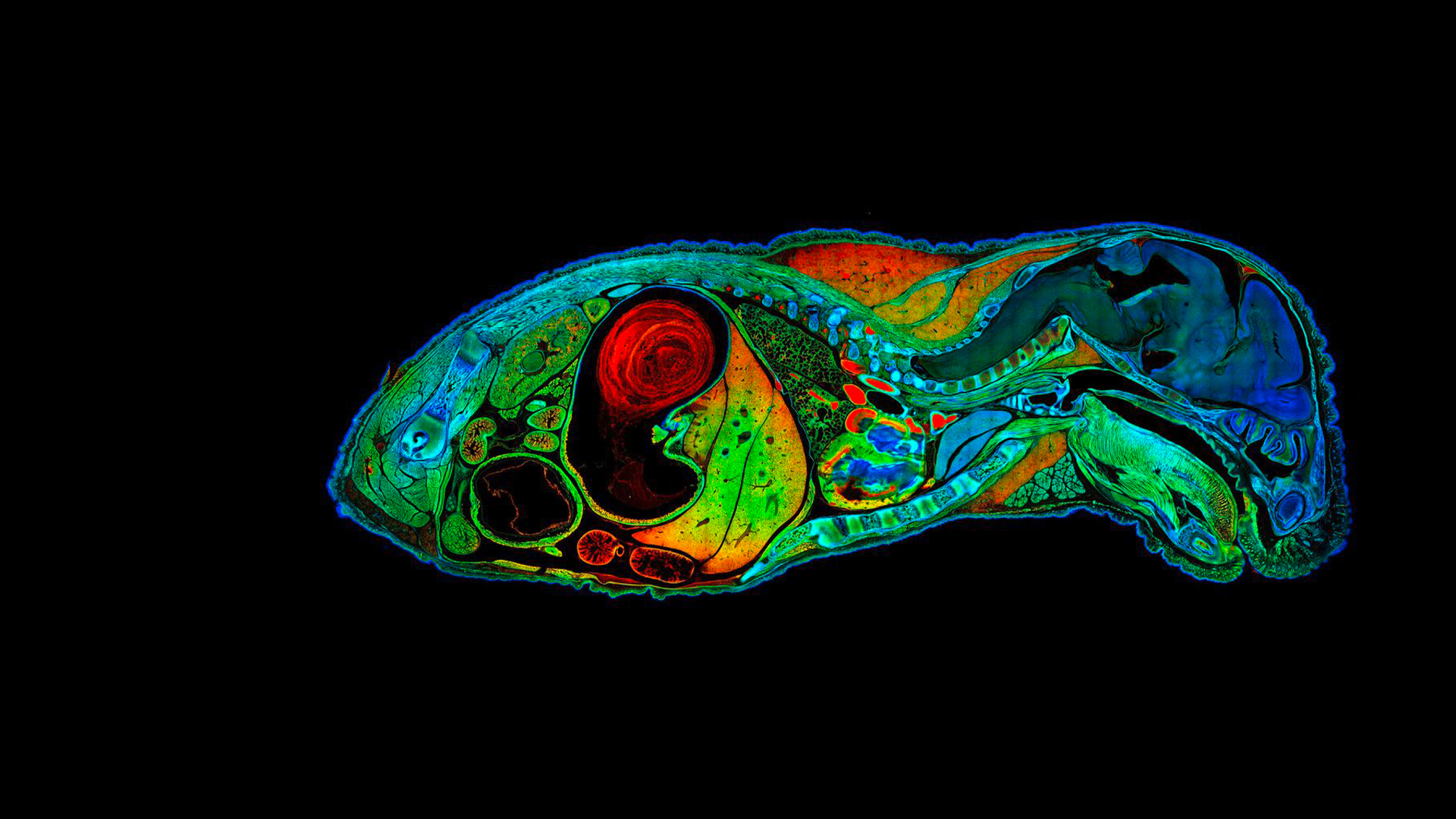
Mouse embryo mosaic image
High resolution mouse embryo mosaic image of 722 tiles containing 190 Megapixels. FLIM data fitted with four characteristic fluorescence lifetimes, color coded. Acquisition: 1:23 h. Analysis: 1:00 h
Follow us on Instagram