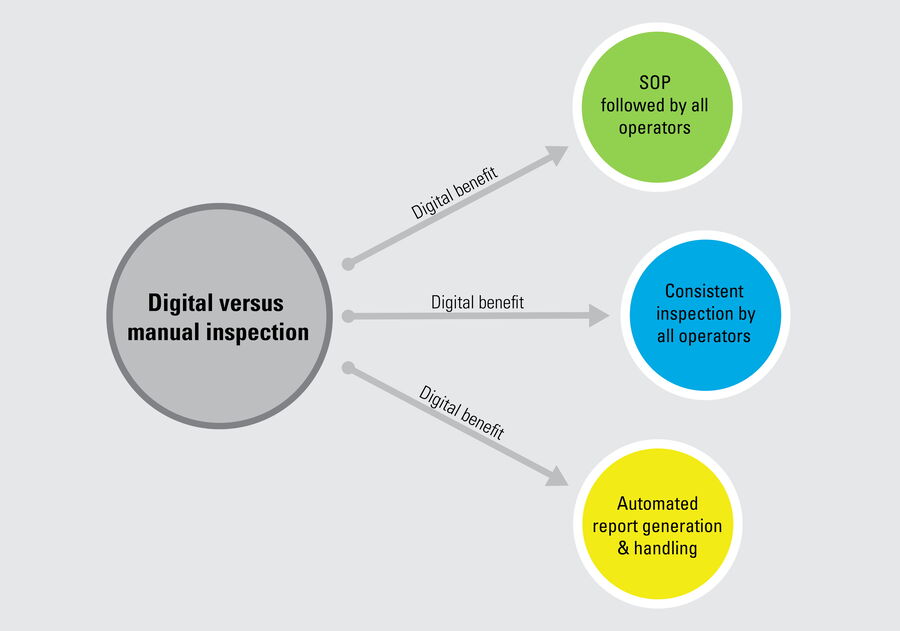
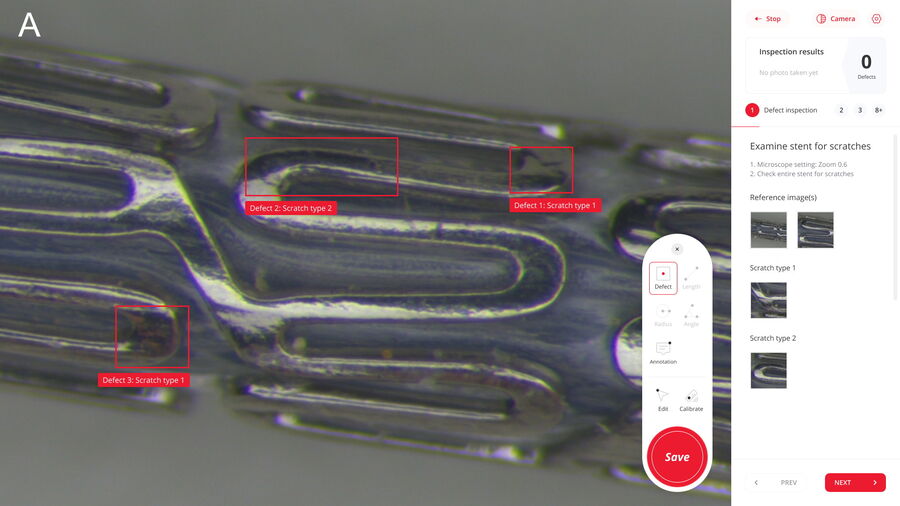
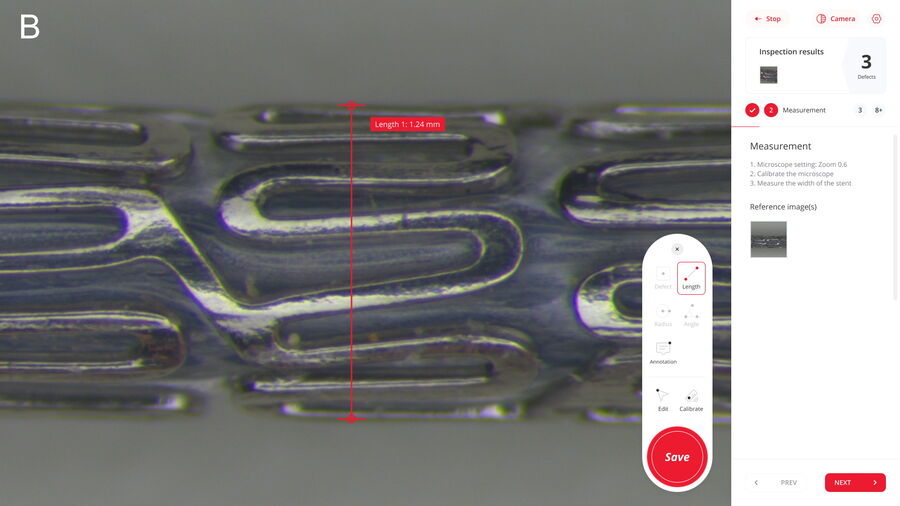
There are multiple reasons why manual inspection can lead to inconsistency. The main ones are the difficulty to ensure that standard procedures are followed by multiple operators doing inspection, too many manual steps, such as data transfer, and paper-based documentation rather than digital. In contrast, a digitally enhanced solution enables more consistent and efficient inspection. To help users achieve this goal, the Exalta smart device for traceable microscopy offers step-by-step work instructions for all operators doing inspection and defect analysis, practical display of reference images and overlays for easy comparison, comparison of results with defined specifications, automated data storage, and digital report generation.
Introduction
Manual visual inspection of product parts and components is often exploited in the medical device industry for quality assurance and control (QA/QC), despite the fact that it can lead to inconsistent results. There are many reasons why manual inspection can be challenging and unreliable. The challenges and how a digitally enhanced inspection solution can overcome them is discussed. Quality managers and operators in the medical device industry can develop such a digital inspection approach with the help of the Exalta smart device for traceable microscopy from Leica Microsystems. The benefits are faster analysis, more consistent and more reliable results compared to commonly used manual solutions.
Manual visual inspection using microscopy
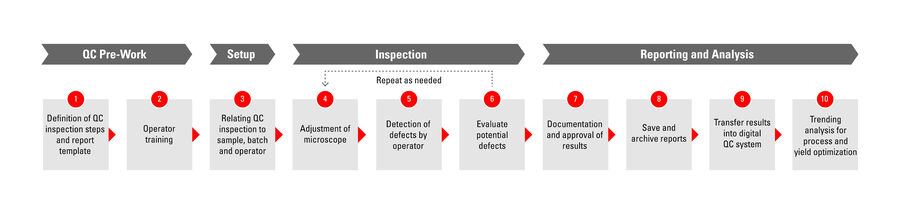
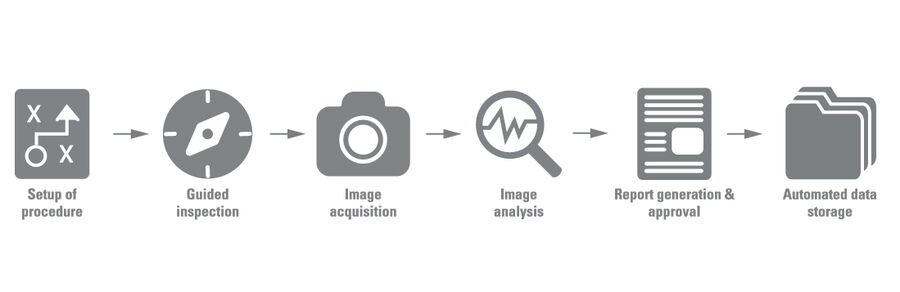
In many cases, manual visual inspection performed with a microscope can lead to inefficient and unreliable results. This disadvantage can occur due to inconsistency at several steps throughout the inspection workflow, from definition and implementation of the standard procedures to the actual device inspection and reporting of results. The steps of a typical manual inspection workflow are shown in figure 1 below.
Challenges faced with manual visual inspection
To better explain how inefficient, inconsistent, and unreliable medical device quality control can arise from manual visual inspection, the challenges faced during manual inspection are sorted into two categories which are described below. These challenges make manual inspection of medical devices less practical and efficient than desired.
1) Implementing inspection workflow steps, training operators, and approving reports
Quality managers define and implement all the procedures for each step of the manual inspection workflow, train operators, and track results, and approve reports (refer to QC Pre-Work, Setup, and Reporting and Analysis steps of Fig. 1). They can encounter the following challenges.
Training operators
Because performing the manual steps of the standard procedures relies on a sort of “honor system” and the operator’s level of experience and expertise (enough to perform the steps correctly), managers can find it difficult to verify if procedures were followed accurately across multiple operators. As a result, managers may end up investing much time and effort training operators so that they become proficient in inspection. These actions are intended to make it more likely that procedures are followed accurately across all operators and consistent results are then obtained.
Implementing and updating procedures
Additionally, there can be different procedures for different product types and regular updates or revisions of procedures during product life cycles. If the different or updated/revised procedures are not manually distributed to every workstation by the manager in a timely way, then there is the risk that operators follow incorrect or outdated standard procedures.
Approving reports
Additionally, often the management of data and report approvals is time-consuming and impractical. Managers may discover that establishing and maintaining a transparent and practical traceability of results, which is required for quality regulations and guidelines, can become awkward and intractable due to cumbersome data and report management. Data documentation and reports can be simply on paper or in very basic, not-easy-to-use databases.
Manual data transfer
When performing manual inspection, results data are not transferred automatically into databases where they can be used for further analysis, managers (or even operators) end up doing it manually which can take a lot of time. As the data transfer is performed manually, it can seriously slow down the entire quality control process and has greater risk of human error, making it inefficient and more costly.
2) Performing inspection
Operators follow the procedures to do the actual manual inspection with microscopes (refer to Inspection step of Fig. 1). Also, they are confronted with several challenges.
Screening of defects
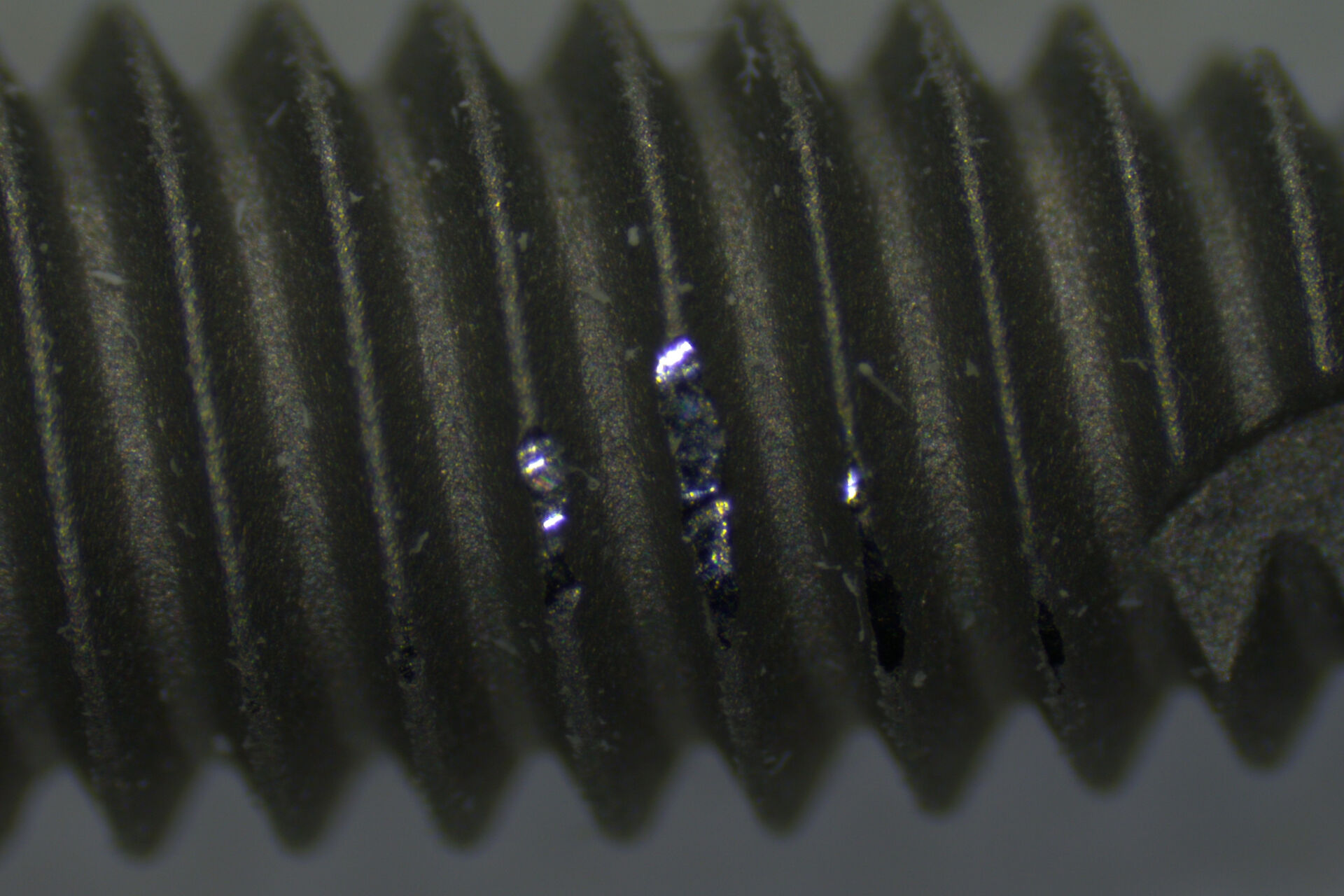
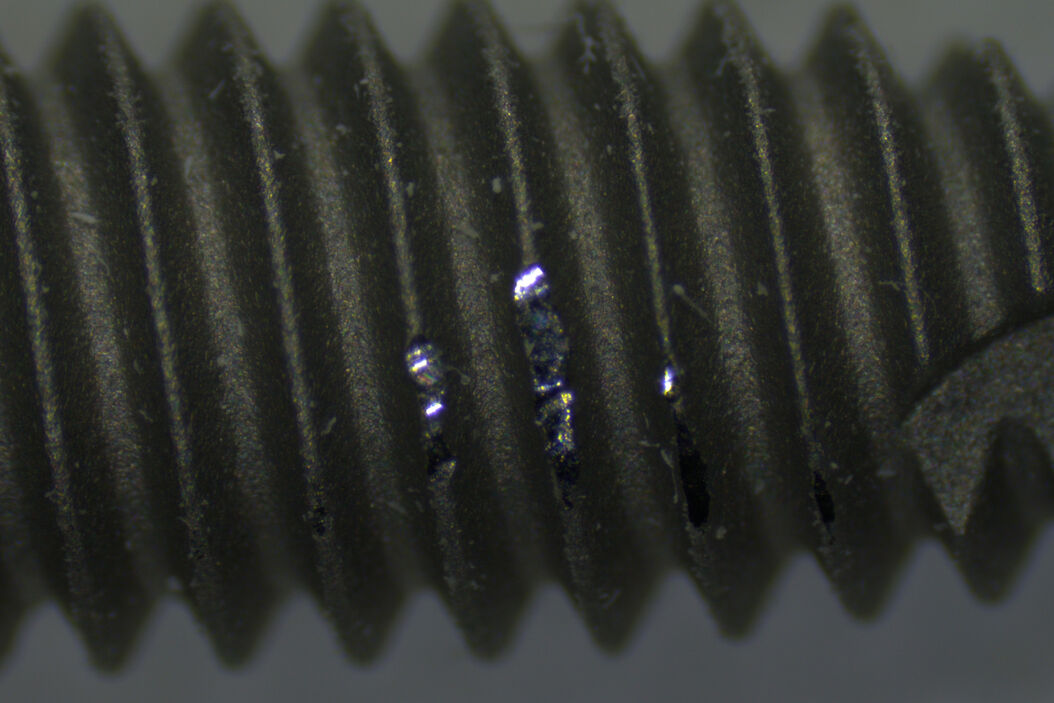
When performing the manual steps of the inspection procedure, there is a significant risk of error. For example, when screening the entire medical device, they may not follow the procedure accurately, overlook relevant areas, and miss defects. Even when screening the device properly, sometimes they may be unable to identify correctly defects which have an effect on the device performance, like damaged or contaminated areas beyond a certain size. Therefore, the accurate screening and identification of defects is important.
Consistent evaluation
Also, when multiple operators are manually inspecting devices, inadvertently they may not evaluate defects in the same way. In other words, how the same defect is evaluated may differ from one operator to the next during manual inspection. This disadvantage is more likely to happen when operators are just starting out. For instance, a new operator may find it cumbersome to efficiently make comparisons with reference images, especially when doing inspection via microscope eyepieces, and need a significant amount of time to learn how do to it accurately.
Manual documenting of results
Finally, as results for defect evaluation and measurement is not stored automatically when performing manual inspection, operators can spend a lot of time documenting it in a manual way, e.g. writing it down on paper. This necessary manual task again increases the chance of human error and can seriously slow down the entire quality control process, making it inefficient and more costly.
Fatigue
As it is common that operators spend long hours performing manual inspection, they can find it difficult to stay focused after long periods of time due to fatigue, particularly when observing medical devices through microscope eyepieces. This fact, again, leads to the risk that they end up deviating from standard procedures and incorrectly evaluating defects.
Overcoming the challenges with a digitally enhanced inspection
Although there are significant challenges related to manual visual inspection of medical devices, a solution does exist that can help users overcome many of them. The answer is a digitally enhanced inspection solution which can be established with the help of Exalta (refer to Fig. 2 and 3). Such a solution offers quality managers and operators the advantages mentioned below.
Fatigue and stress, which can occur with long manual inspection times via the eyepieces, can be reduced with digitally enhanced inspection, because it is done with a microscope using a digital camera, monitor display, and available software having features for analysis.
The manager can define digital step-by-step work instructions and rapidly implement them, so they are available to all microscope workstations, therefore helping to ensure inspection is done in accordance with the defined standard procedures. This is possible even when multiple operators are inspecting the same or different parts and components, enabling more consistent screening of defects. Moreover, with instructions for defect analysis, including distinguishing, measuring, and counting defects, a more accurate evaluation of defects can be achieved. As a result of these benefits, training of new operators can require substantially less time and effort.
There is also a practical display of defect reference images and overlays for easy, rapid comparison. An automatic comparison of results with defined specifications enables faster confident pass/fail decisions and can lead to more consistent screening and evaluation of defects. The digitally enhanced solution offers the automatic storage of inspection data, e.g. from defect evaluation, and automated transfer into a database. This capability helps to minimize manual tasks, like documenting results and transferring data, and allows more straightforward traceability of results as well as generation and approval of reports.
Summary and conclusion
Manual visual inspection of medical devices can be inconsistent and inefficient. Results can vary between operators as defined standard procedures may not be accurately followed. Additionally, often there are time-consuming manual steps like documenting results and transferring data into a database. A digitally enhanced visual inspection solution can be developed with the aid of Exalta, a smart device for traceable microscopy from Leica Microsystems. The digital approach helps operators and managers to overcome the issues of manual inspection for improved consistency, reliability, and efficiency. Additionally, as the traceability of results and approval of reports are simpler compared to manual inspection, audits concerning regulations like 21 CFR Part 11 and GMP Annex 11 are done more easily.