Critical point drying – an introduction
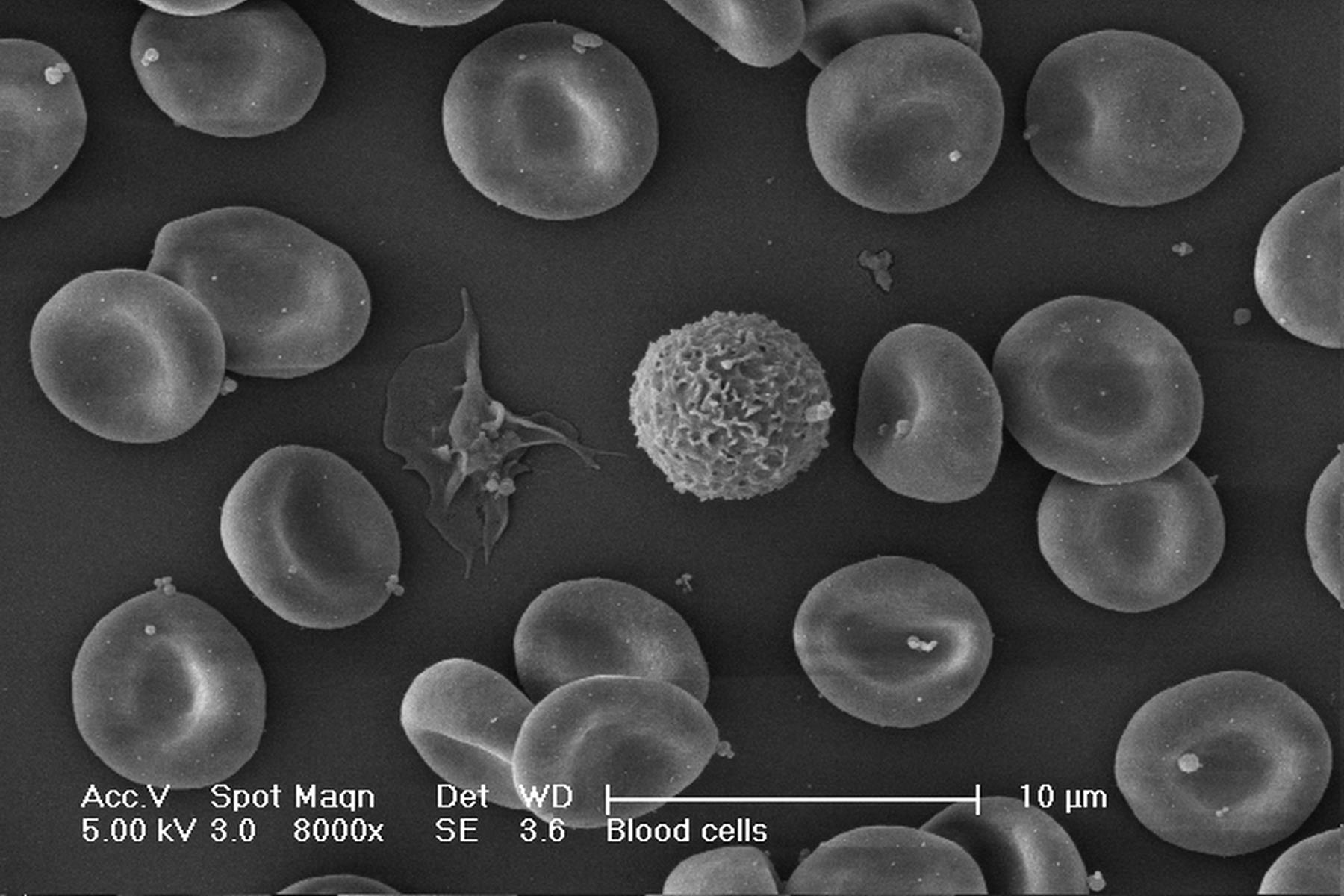
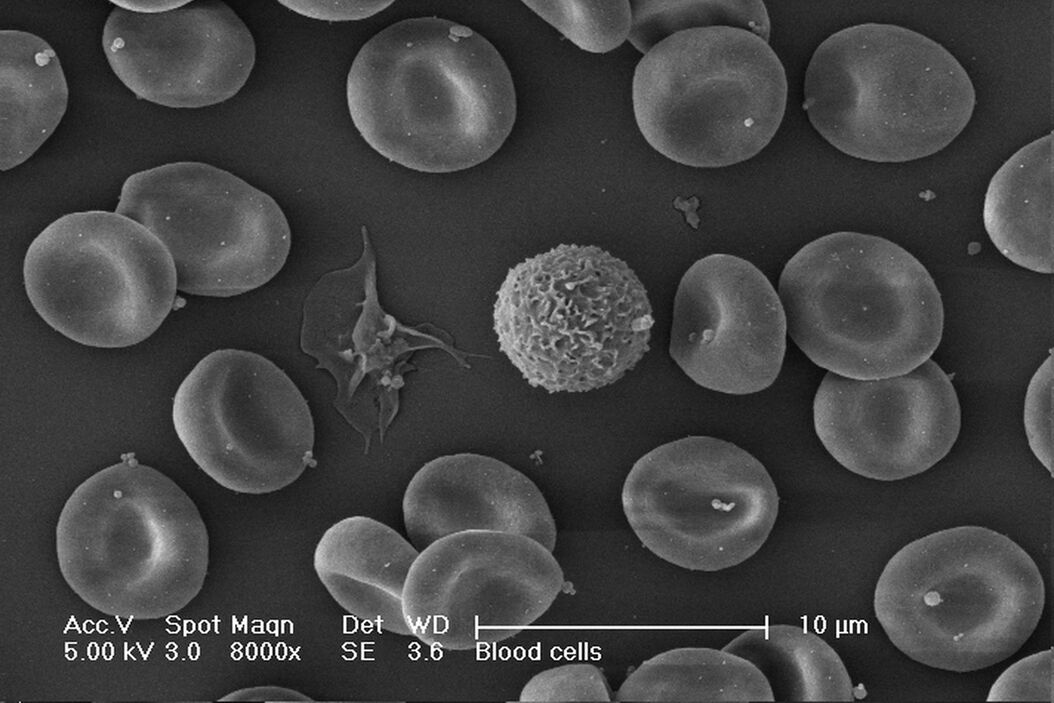
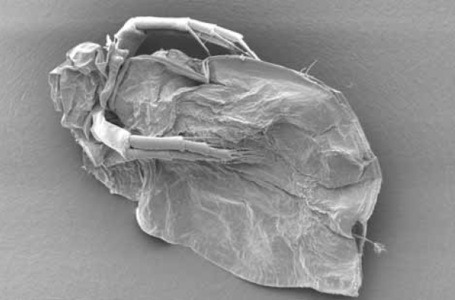
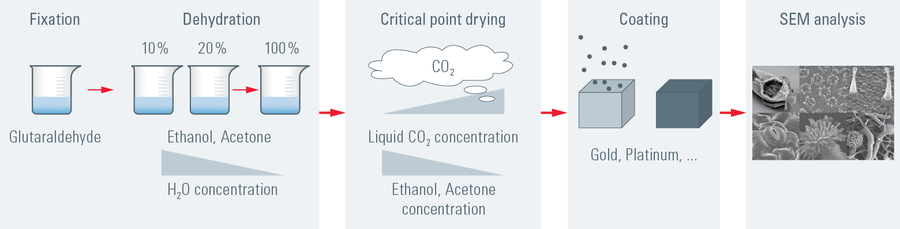
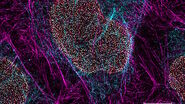
One of the uses of the Scanning Electron Microscope (SEM) is in the study of surface morphology in biological applications which requires the preservation of the surface details of a specimen. Samples for Electron Microscopy (EM) imaging need to be dried in order to be compatible with the vacuum in the microscope. The presence of water molecules will disturb the vacuum and with it the imaging. It will also cause massive deformation or collapse of the structures under investigation (see "Comparison between air and critical point drying"). Water has a high surface tension to air. Crossing the interfaces from liquid to gaseous phase during evaporation (air drying) the tangential forces caused by the surface tension can have an effect on the nano and micro structures of the specimen.
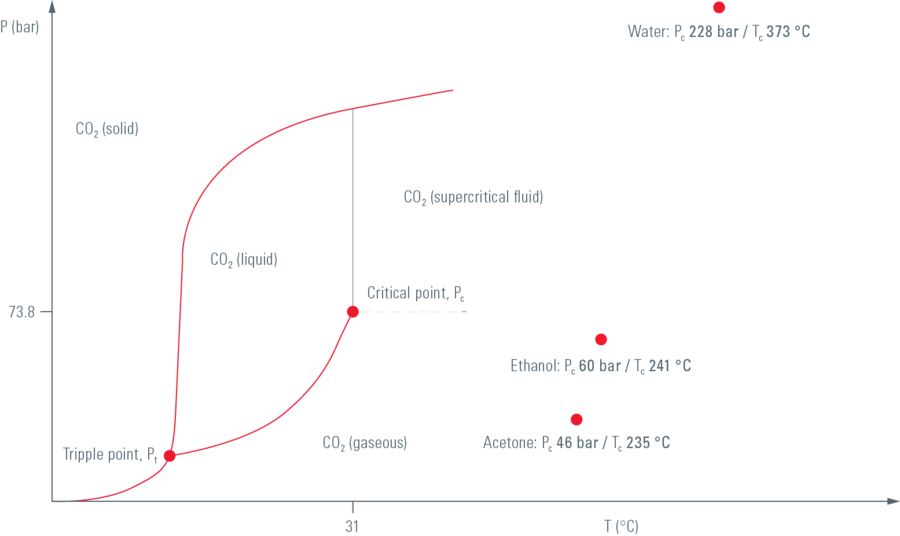
To preserve sample morphology, critical point drying is the state of the art method (see "Pressure/temperature phase diagram for CO2"). At the critical point physical characteristics of liquid and gaseous are not distinguishable. Compounds which are in the critical point can be converted into the liquid or gaseous phase without crossing the interfaces between liquid and gaseous avoiding the damaging effects. The dehydration of the samples using the critical point of water is not feasible since it lies at 374 °C and 229 bar where any biological sample would be destroyed. To overcome this problem, water can be replaced against liquid carbon dioxide (CO2), whose critical point lies at 31 °C and 74 bar and is more appropriate for all biological applications and technically relative easy to maintain.
However, CO2 has one serious disadvantage as transitional fluid; it is not miscible with water. Therefore, water has to be replaced by exchange fluids like ethanol or acetone which are miscible in both water and liquid CO2. Both exchange fluids can not be used for critical point drying due to their high critical point temperatures (Ethanol: Pc 60 bar/Tc 241 °C; Acetone: Pc 46 bar/Tc 235 °C). After replacing water with an exchange fluid in a pre-critical point drying step and in turn replacing this exchange fluid with liquid CO2, the liquid CO2 is brought to its critical point and converted to the gaseous phase by decreasing the pressure at constant critical point temperature.
Comparison between air and critical point drying
Automated processing
Downloads
Download the "Application Booklet" and read more about standard protocols to facilitate the optimizing process of critical point drying protocols and the use of the automated critical point dryer Leica EM CPD.
Download the brochure "EM Sample Preparation – Critical Point Drying"