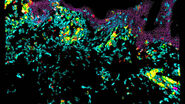
Advantages of an open-top multisample dual-view light sheet microscope
The paper focuses on the Viventis Deep microscope from Leica Microsystemsthat is designed for high-resolution live imaging of large multicellular systems. The microscope combines dual-view and dual illumination configurations. This allows detailed visualization of the in toto dynamics of organoids. An example in the paper shows how the dual detection allows the visualization of individual cells throughout the sample volume. In this way the light sheet arrangement in this microscope offers several key advantages for researchers in the field.
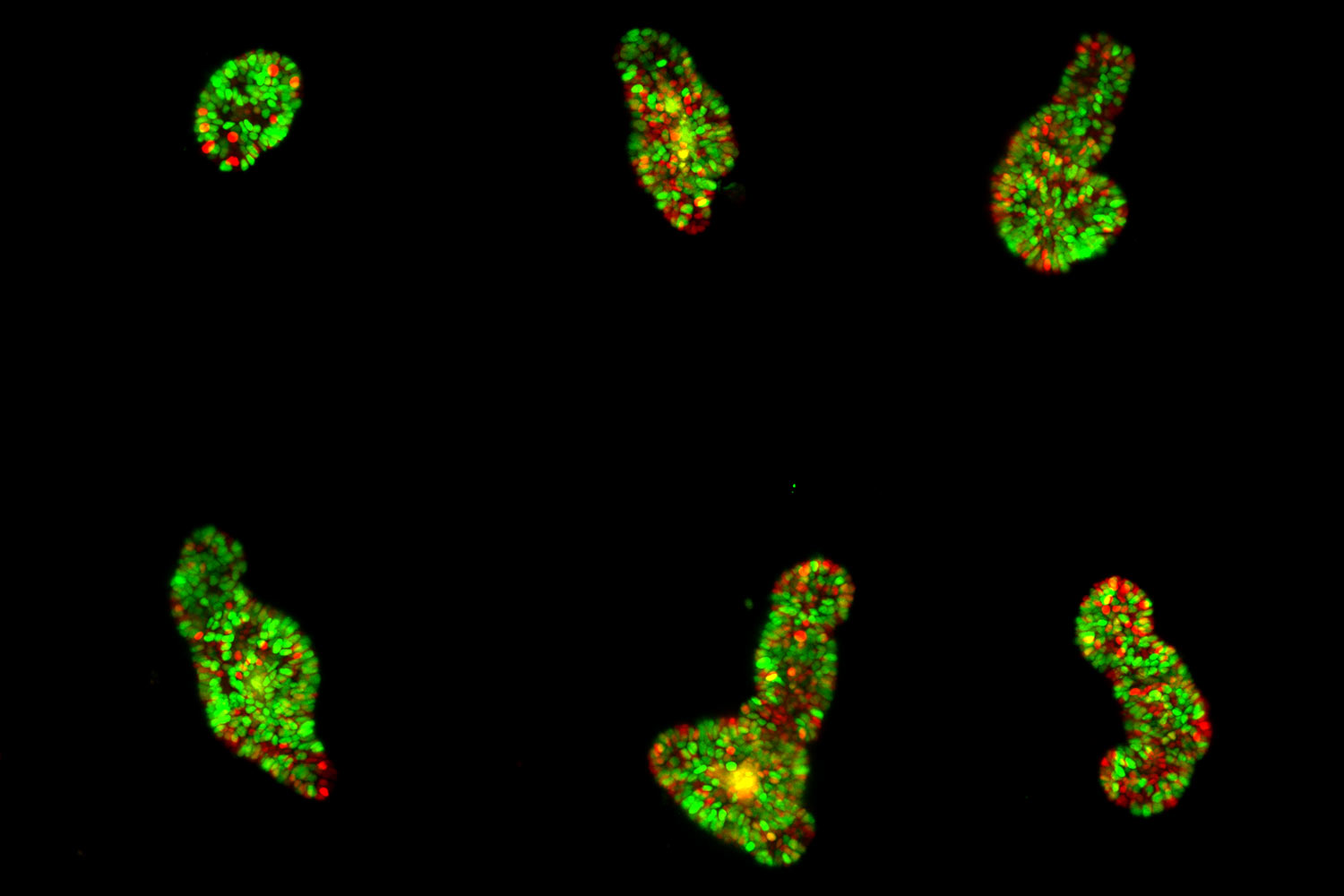
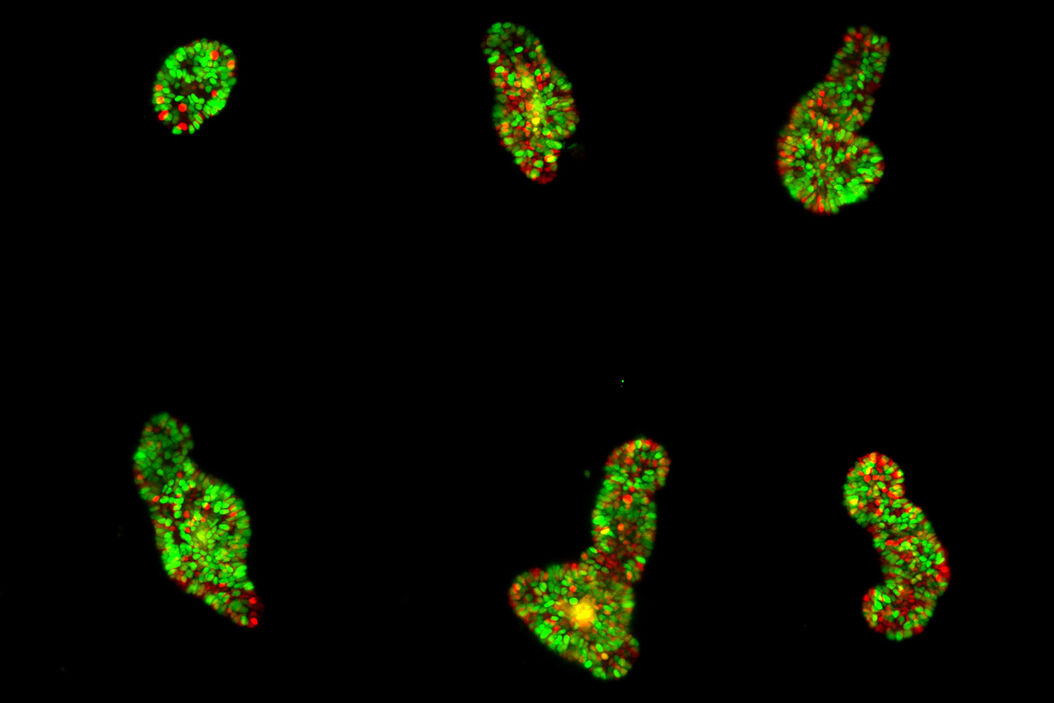
The authors highlight the successful imaging of various specimens, including mature intestinal organoids, gastruloids, Hydra, and human colon cancer organoids. These large specimens, up to 550 µm in size, were imaged continuously for up to 12 days, providing valuable insight into their dynamic behavior.
Thanks to a unique 3D arrangement of the illumination and detection objective pairs, the system provides space for an open-top sample holder with an array of up to four sample chambers. It combines the 3D imaging capabilities of light-sheet microscopy with multi-position imaging. It enables robust conclusions even in the presence of sample-to-sample heterogeneity. In addition, the chambers allow top access for pipetting even during the experiment.
Tracking cells over time and across scales
The authors show how they leveraged the microscope's unique capabilities. In one of their experiments, they follow the development of maturing mouse intestinal organoids withthe goal of observing crypt and villus formation over a three-day period. The microscope's exceptional resolution and temporal capabilities provide detailed insights into single-cell dynamics within these large specimens.
The versatility of the microscope is shown by imaging mouse liver and human colon cancer organoids. Other types of samples used by the authors include murine parotid salivary gland organoids and gastruloids. In each case, the microscope consistently delivers high-quality images while maintaining the natural growth and environmental conditions required for accurate scientific analysis.
Using advanced tracking, segmentation, and immunofluorescence techniques, the microscope enables high-quality single-cell data acquisition. The authors of the paper provide examples of the valuable insights gained into the behavior and maturation processes of different cell types within the imaged samples. The combination of live imaging and immunofluorescence within the entire organoid volume proves instrumental in unraveling complex cellular dynamics.
Read the full article or visit the product page for more information
To learn more about the open-top, multi-sample, dual-view light-sheet microscope, read the open-access publication at Nature Methods.
Additional information about the Viventis Deep microscope can be found on the product page for a deeper understanding of its technological advances and benefits for live imaging of large multicellular systems.
Open-top multisample dual-view light-sheet microscope for live imaging of large multicellular systems
Franziska Moos, Simon Suppinger, Gustavo de Medeiros, Koen Cornelius Oost, Andrea Boni, Camille Rémy, Sera Lotte Weevers, Charisios Tsiairis, Petr Strnad and Prisca Liberali
Nat Methods (2024). doi.org/10.1038/s41592-024-02213-w