Follicular lymphoma presents a complex clinical picture. Some patients have a slow progressing illness, while others relapse quickly after initial treatment with poorer prognosis. There is currently no predictive toolset to differentiate early relapsing patients from slower progressing patients. To solve this issue, and potentially improve care for early relapsing patients, the authors used a multi-omics spatial biology approach that leveraged bulk RNA-seq, single-cell RNA-seq, Iterative Bleaching Extends Multiplexity (IBEX) imaging, and an adaptation of the Cell DIVE process using IBEX dye inactivation chemistry (Cell DIVE-IBEX). The authors also made use of computational tools such as analysis of cellular communities to understand the spatial positioning of cell phenotypes within normal and diseased lymph nodes. Using these tools, a clearer picture of early relapser follicular lymphoma emerges. Lymph nodes from these patients have smaller follicles, spaced further apart, and exhibit increased stromal cell populations with evidence of desmoplasia. Using these genetic, histological, and spatial characteristics, researchers and clinicians may, in the future, be able to stratify patient populations based on risk and make informed research and treatment decisions. Overall, this study shows the power of the multi-omics, spatial biology approach, and indicates how flexible multiplexing with Cell DIVE can be used to enhance the power of an immune-oncology study.
About the Case Study
Key Learnings
- Learn the molecular and spatial details of follicular lymphoma and how it manifests in different patient groups
- Discover how sequencing of RNA molecules can be combined with multiplexed imaging to reveal the complex phenotypes of cells in the tumor microenvironment
- Understand how the Cell DIVE process can be adapted to fit different laboratory needs and dye inactivation chemistries
- See an example of how complex multi-omics data can be analyzed to yield concrete, actionable results in the immune-oncology space
Introduction
Follicular lymphoma (FL) is an incurable cancer arising from germinal B cells located within lymph nodes. It is a cancer with a complex clinical course—in some patients the disease progresses very slowly, while in some patients, termed “early relapsers”, the disease progresses quickly after an initial run of treatment. Currently, there is no predictive toolset available to identify early relapsers. To characterize the spatial and genomic characteristics of early relapsers, here, the authors made use of multiple sequencing and imaging methodologies to create a multi-omics profile of FL. Iterative multiplexing with Cell DIVE was used to expand this analysis to large, whole-tissue sections to improve the power of the study. They report fine details of how follicular histology is altered in early relapsers, and find that desmoplasia, extracellular matrix deposition, and stromal cell growth are strongly associated with rapid progression of FL.
Results
To begin, the authors used bulk RNA sequencing on both normal and diseased lymph node tissues, which contained non-progressors, progressors within 2 years, and early relapsers. This analysis revealed alterations in the expression of chromatin modifiers, cell migration factors, and immune regulators in the tumor samples. A deconvolution algorithm was then applied to identify cell types from the sequencing data and revealed that most cells in the analysis were B cells, with minor myeloid and stromal subpopulations. Single-cell sequencing was able to further define cell subtypes within the greater immune groups. Together, these genomic approaches found that pathways upregulated in early relapsers were related to B cell receptor signaling, cytokines, immune activation/regulation, as well as extracellular matrix remodeling.
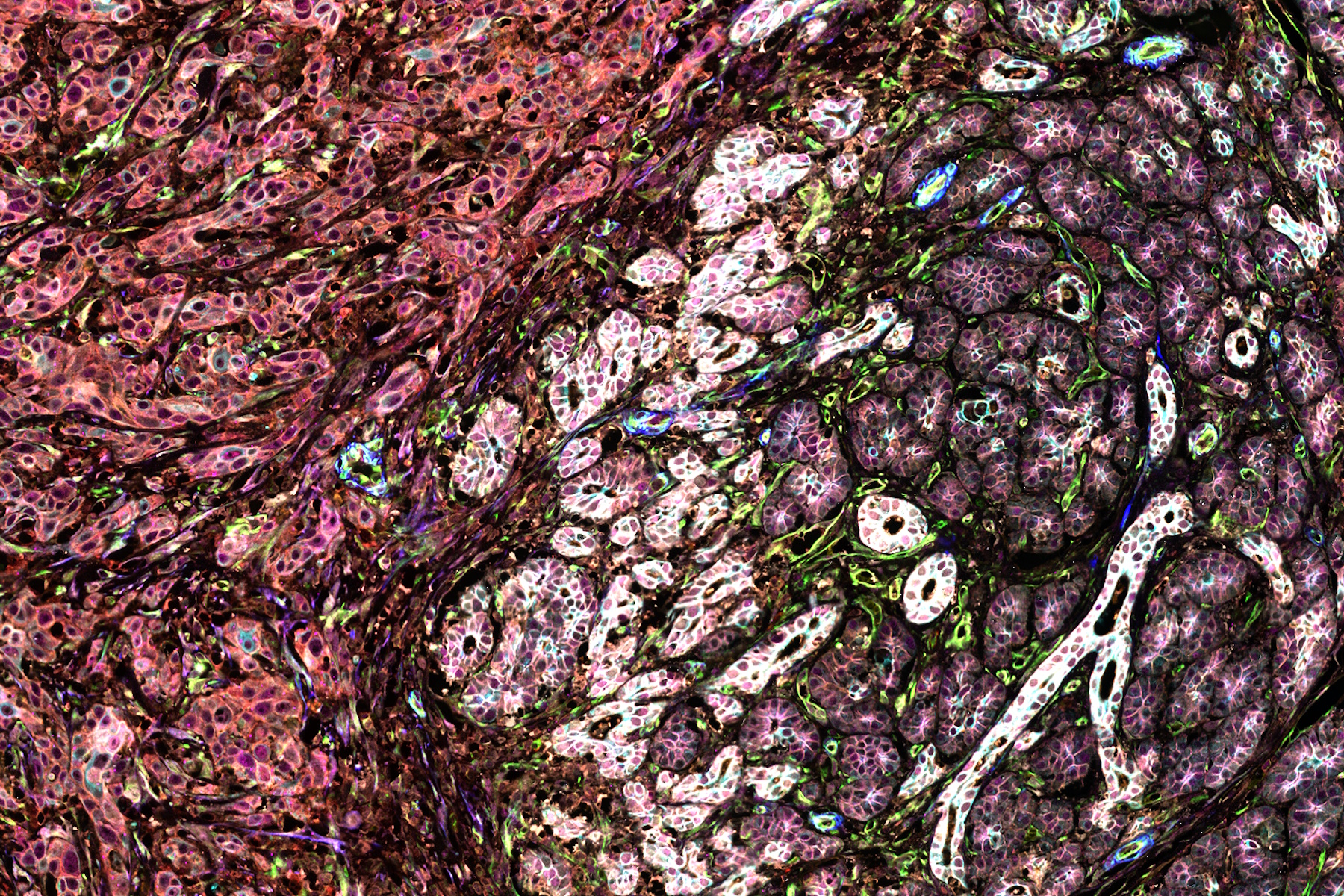
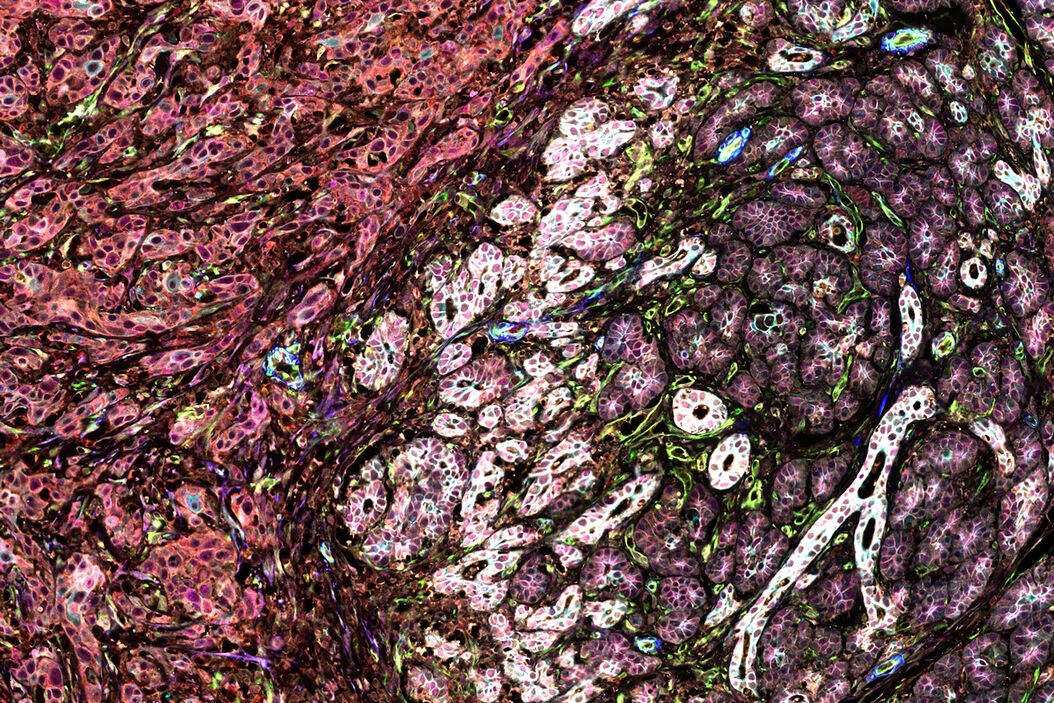
To characterize the FL tissues further, the authors then applied a multiplexed imaging methodology known as IBEX (Iterative Bleaching Extends Multiplexity) to visualize a 40-plex biomarker panel across normal and FL tissue. Cells in the resultant images were segmented using membrane markers and a deep learning approach. Clustering analysis with a UMAP algorithm allowed phenotyping of the over 1 million cells in the analysis and revealed a diversity of B and T cell phenotypes across the FL tissues. Owing to their complex morphology, myeloid and stromal cells were difficult to segment, and thus specialized segmentation methods were developed to analyze these cell types. Early relapser tissue examined using IBEX revealed distinguishing patterns in follicle organization. The authors found desmin-positive fibroblasts around B cell follicles, as well as DC-SIGN-positive cells within the follicles, two histological findings correlated with poorer prognosis.
The authors used data from the IBEX and RNA-sequencing approaches to create an FL reference atlas, which allowed for direct comparison of the two techniques. They found that the overall characterization of lymphocytes in the two methods agreed well, but that the RNA-sequencing approach under-represented myeloid and stromal cells. The multiplexed imaging approach with IBEX also involved a larger cell count, which offered utility in finding and identifying rarer subtypes that might be overlooked using sequencing. RNA-sequencing was also able to identify cytokine transcripts, which overall agreed well with the imaging data.
To add power to the analysis, the authors next turned to Cell DIVE multiplexed imaging to expand the patient cohort. A novel alteration of the traditional Cell DIVE dye inactivation protocol was used, and the authors used IBEX inactivation reagents to perform the staining, destaining, and imaging of a 11-plex immune and 6-plex stromal panel to quantify and analyze follicles in whole tissue sections. The authors used this technique to examine the morphology of follicles in early relapsers, finding generally that the follicles of these patients were smaller, more irregular, and further apart than slower progressing patients.
Overall, the authors found several distinguishing morphological, genetic, and cellular alterations that occur in early relapsing FL patients that could be used to identify these patients earlier in the course of treatment. In addition to the changes observed in the size and spacing of follicles, a general decline in B cell communities, as well as expansion of stromal cells and changes to the extra-cellular matrix were observed. These findings offer the possibility of improved clinical and pre-clinical stratification of patients, more informed treatment decisions, and suggest new targets for antibody-based therapies such as anti-desmin and anti-vimentin drugs. This study, drawing predictive power from sequencing, IBEX imaging, and Cell DIVE, allowed for the deep characterization of this complex disease and points the way to a new approach for FL.
Abstract link
Full article:
Radtke AJ, Postovalova E, Varlamova A, Bagaev A, Sorokina M, Kudryashova O, Meerson M, Polyakova M, Galkin I, Svekolkin V, Isaev S, Wiebe D, Sharun A, Sarachakov A, Perelman G, Lozinsky Y, Yaniv Z, Lowekamp BC, Speranza E, Yao L, Pittaluga S, Shaffer AL 3rd, Jonigk D, Phelan JD, Davies-Hill T, Huang DW, Ovcharov P, Nomie K, Nuzhdina E, Kotlov N, Ataullakhanov R, Fowler N, Kelly M, Muppidi J, Davis JL, Hernandez JM, Wilson WH, Jaffe ES, Staudt LM, Roschewski M, Germain RN:
Multi-omic profiling of follicular lymphoma reveals changes in tissue architecture and enhanced stromal remodeling in high-risk patients
Cancer Cell. 2024 Feb 21:S1535-6108(24)00045-X. doi: 10.1016/j.ccell.2024.02.001. Epub ahead of print. PMID: 38428410.