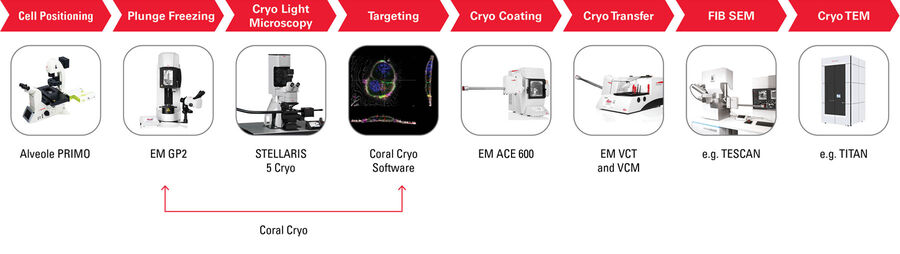
Cryo Electron Tomography (CryoET)
Cryo Electron Tomography (CryoET) is used to resolve biomolecules within their cellular environment down to an unprecedented resolution below 1 nm. In this way, individual proteins can be recognized by their shape alone, without any labelling. Even different conformations can be distinguished, opening a window to truly understanding molecular sociology.
By opening up new approaches to understanding spatial protein structures and interactions, CryoET imaging helps to decode cellular mechanisms and translate these insights. For example, they may help to find effective treatments for many diseases.
But imaging at subnanometer resolution comes with major challenges: it is cost intensive, has a high training effort, involves many instruments steps and mostly the structures of interest cannot be selectively visualized in the electron microscope (EM). To secure a high success rate, the target volume for the EM imaging therefore needs to be identified and located with the highest possible resolution during the light microscopy (LM) stages of the workflow.
The seamless Cryo-Electron Tomography workflow Coral by Leica Microsystems uses confocal super resolution for precise targeting. The workflow not only reduces the number of steps for sample preparation but also improves the sample loading and transfer success rate, thereby enhancing the productivity of the On-Grid Lamella/CryoET workflow.
The target coordinates are provided in an open format for the subsequent FIB milling and LM-EM correlation. As a result, you achieve a higher productivity and better reproducibility for your experiments. This allows to accelerate the deployment of the protocols in your facility and have more confidence with your end results.
High precise 3D volume targeting
A STELLARIS 5 Cryo confocal microscope, including cryo stage and shuttle, is the backbone for the precise targeting in the Coral Cryo workflow. Confocal microscopes are superior for 3D targeting compared to widefield instruments by design as they offer a better z-resolution.
With the super resolution data for subsequent high precision correlation, the STELLARIS 5 Cryo as a stand-alone component outperforms EM solutions that are using integrated light microscopes for targeting, and also frees up valuable EM instrument time.
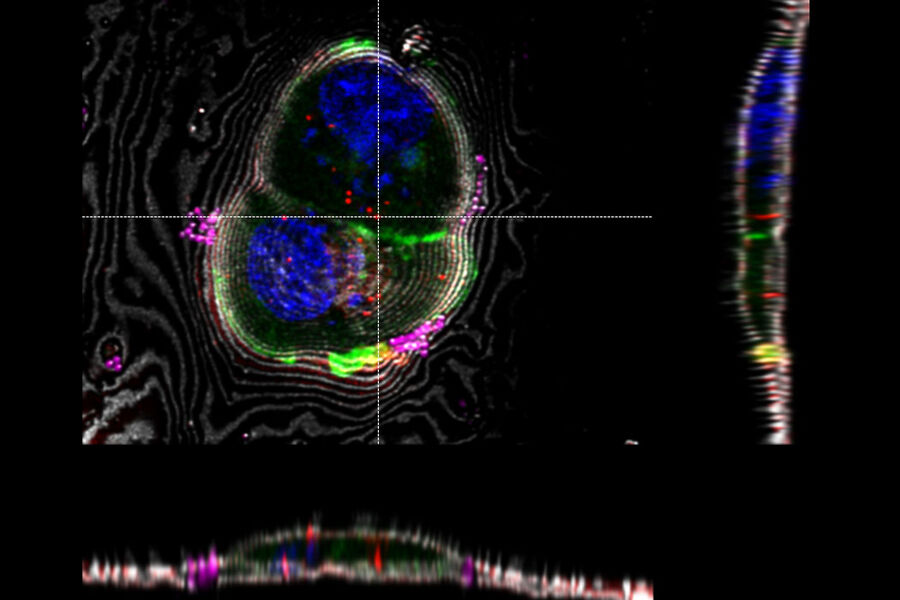
A fiducial marker is applied using fluorescent beads on the sample. The determination of their position within the 3D image data can be used to significantly increase the targeting precision on the z axis. In addition, Leica has also developed an interpolation-based method to find the bead center with even higher precision than the actual optical resolution of the system (patent pending).
Moving through the workflow stages safely
The STELLARIS 5 Cryo is designed to be used in conjunction with state-of-the-art CryoET targeting software (LAS X Coral Cryo), as well as a variety of seamless integration and transfer options to cryo FIB or VCT stages.Workflows for cryo-electron tomography used to contain multiple complex steps, leaving too many margins for error that often resulted in ruining the final all-important imaging step. Manual sample preparation, handling and transfer steps can be tricky and tedious, possibly leading to sample contamination or devitrification.
The Coral Cryo workflow changes this. By streamlining the workflow, this will help to enable more widespread adoption of CryoET. The reduced number of steps and unmatched flexibility and safety ensure sample viability, quality checks and most of all, a precise and reliable 3D targeting mechanism.
The cryo stage is a more stable closed system for easier handling and does not require taking off the cooling insert from the stage. The easy loading process of the sample transfer shuttle minimizes the risk of sample loss and facilitates the transfer along the instrument stages.
The STELLARIS 5 Cryo is designed to be used in conjunction with state-of-the-art CryoET targeting software (LAS X Coral Cryo), as well as a variety of seamless integration and transfer options to cryo FIB or VCT stages.
Benefits against an integrated solution
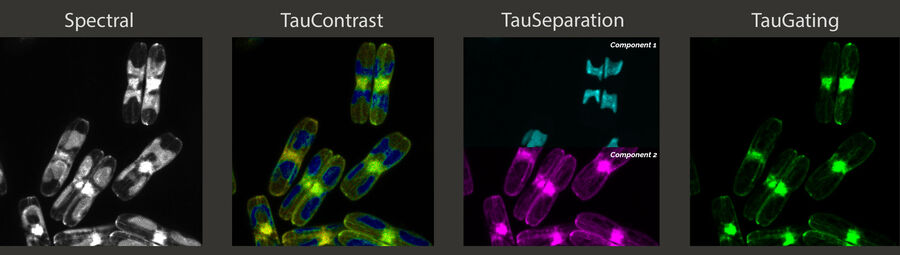
Without an exact location for the target, the approach of the lamella target area is usually a time-consuming iterative approach. In comparison to an integrated LM-EM version, using the Coral Cryo workflow with a STELLARIS 5 Cryo confocal microscope offers higher resolution, better image quality and higher z-resolution through confocal techniques. Coral Cryo offers spectral flexibility for the precise detection of target fluorescence and our unique TauSense technology that removes unwanted autofluorescence and finetunes target identification that can be checked up front before moving on to the EM stages.
The ultimate in flexibility
Coral Cryo offers two different workflow solutions to provide maximum flexibility to the cryo-FIB microscope. With our flexible workflow our cryo transfer system EM VCT can connect to the all cryo-FIB solutions on the market providing you all advantages of the Coral Cryo workflow. For Thermo Scientific Aquilos users we provide our integrated solution reducing the workflow steps even further by hardware integration of our sample cartridge in the Aquilo cryo FIB.
The streamlined end-to-end Coral Cryo workflow offers clearly defined steps, modules and seamless hardware and software interfaces. Coral Cryo can be rapidly deployed in your facility or laboratory thanks to seamless hardware and software interfaces.
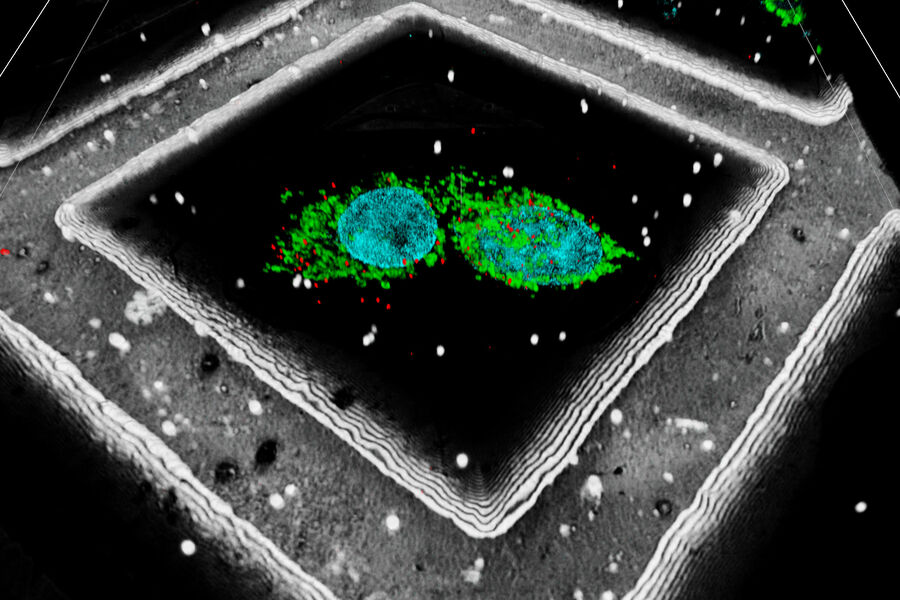
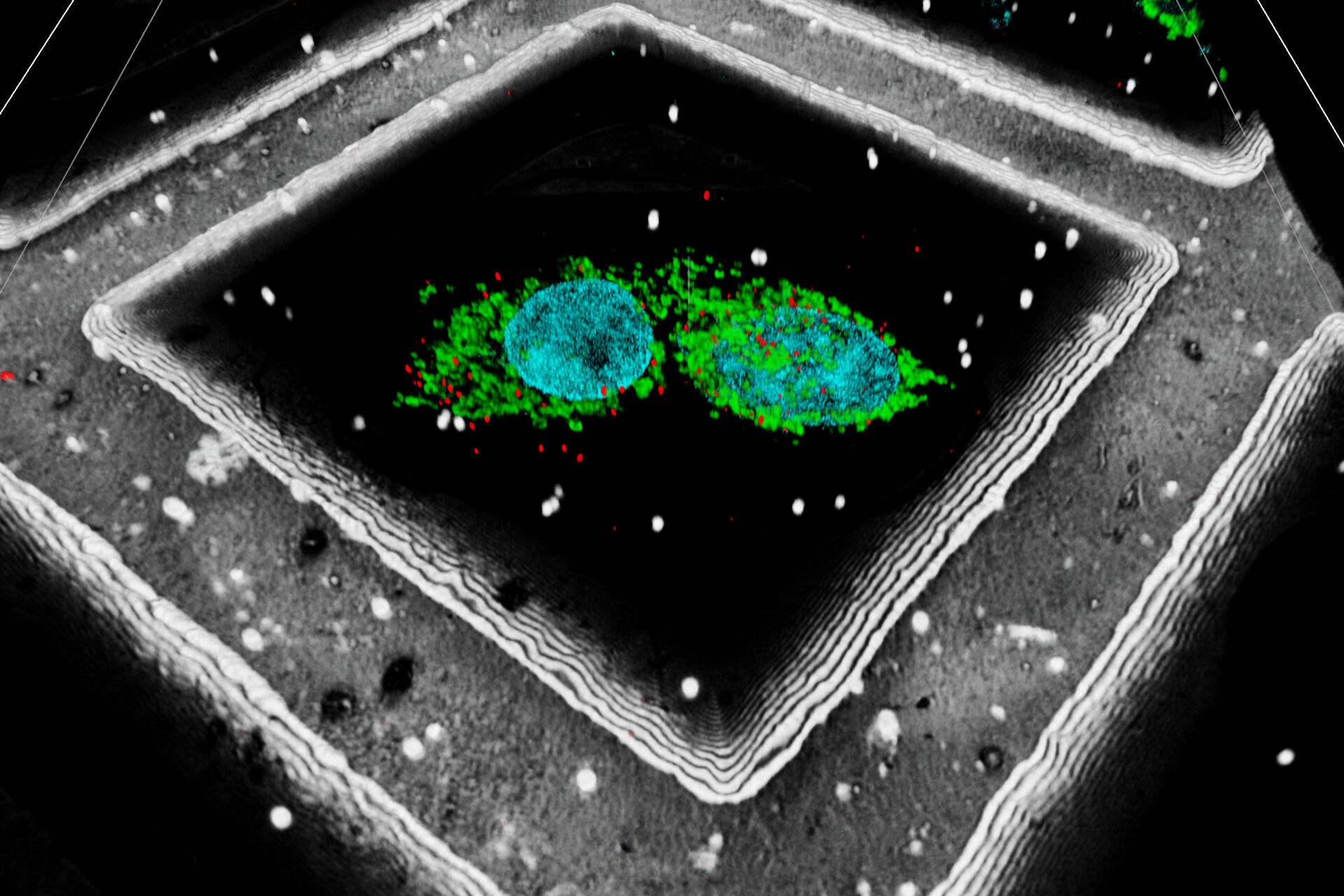
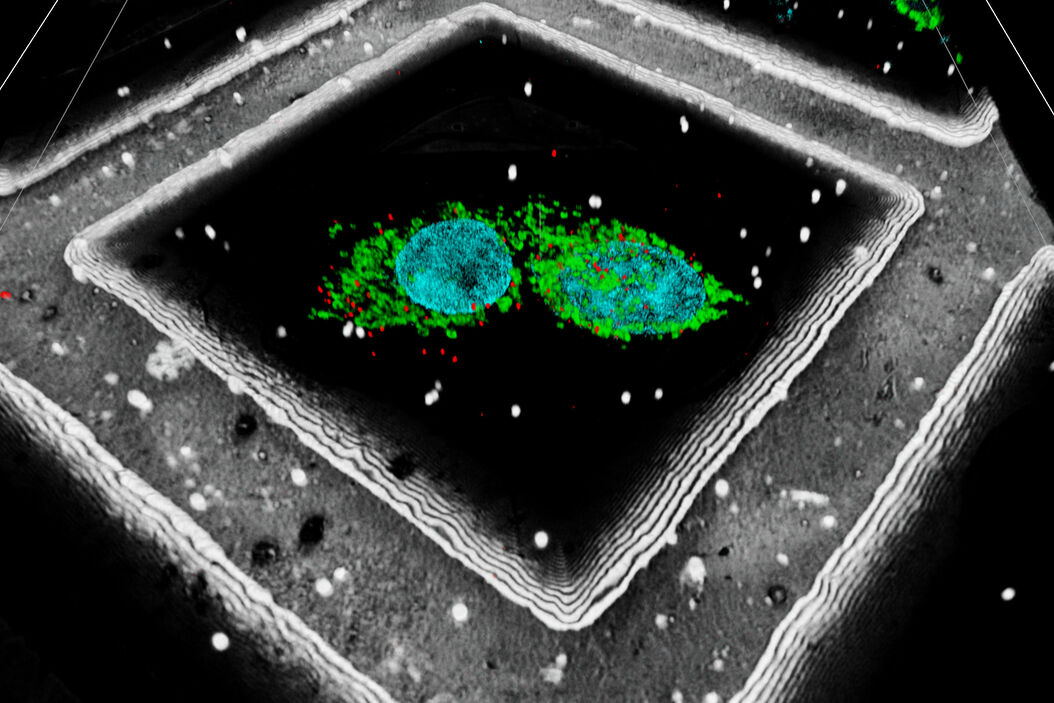
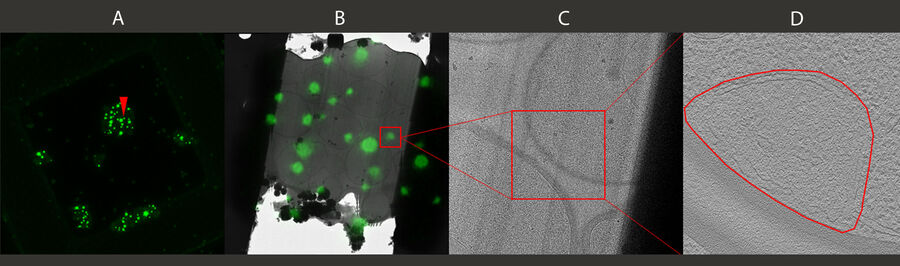
3D targeting of fluorescent structures for cryo electron tomography
A fluorescent structure is identified via super resolution cryo confocal microscopy. Using the beads surrounding the sample as correlation markers the target structure can be identified on the cryo FIB-SEM and a thin lamella containing the target structure can be milled from the cellular volume. The thin lamella is transferred to a cryo TEM and a tilt series of the lamella is recorded. From the tilt series a 3D volume can be calculated down to subnanometer resolution and a rendering created for visualization and analysis.
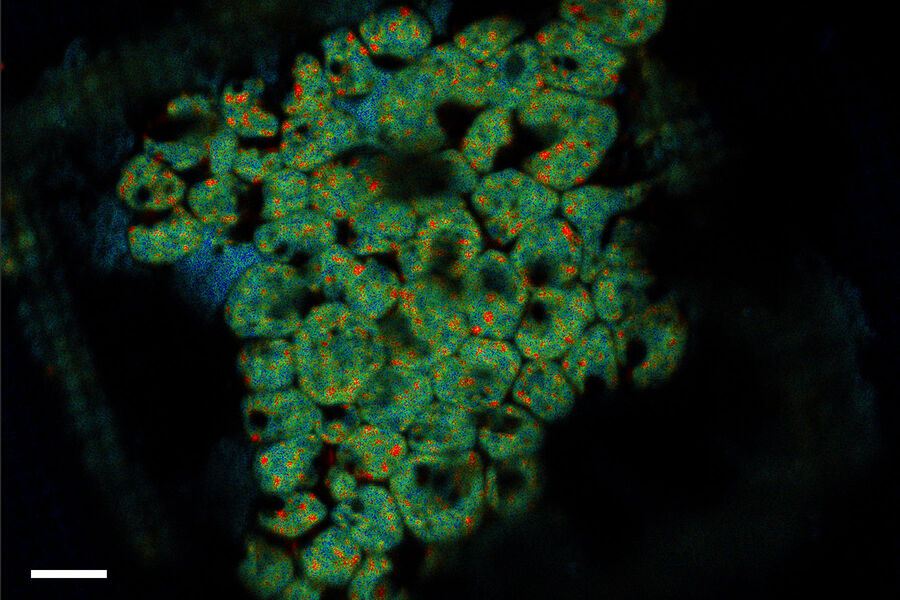
- A - Maximum intensity projection of the confocal 3D volume. The target structure (yeast cell expressing Ede1-eGFP, being involved in clathrin-mediated endocytosis) is depicted by an arrow.
- B - FIB lamella in the TEM after FIB milling, overlay of Ede1-eGFP fluorescence.
- C - Exemplary image of the tomogram of the lamella volume. The square indicates the position of image D.
- D - Detail of tomogram depicting the phase separated endocytotic protein deposit.
Scale: A - Inner grid square width: 90 µm, bar width: 35 µm. B, C, D - Edge length of insert is 2 µm.