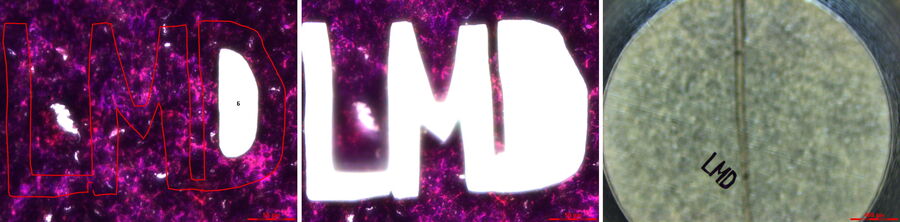The Beginning
The very first Laser Microdissection system was invented already 45 years ago back in 1976 by German pathologists [1,2]. They used an upright Leitz-Orthoplan microscope and already discovered gravity as collection method. The technology was far ahead of time and seemed to be forgotten over time. Maybe because there was no suitable downstream method available to analyze such tiny samples as single cells.
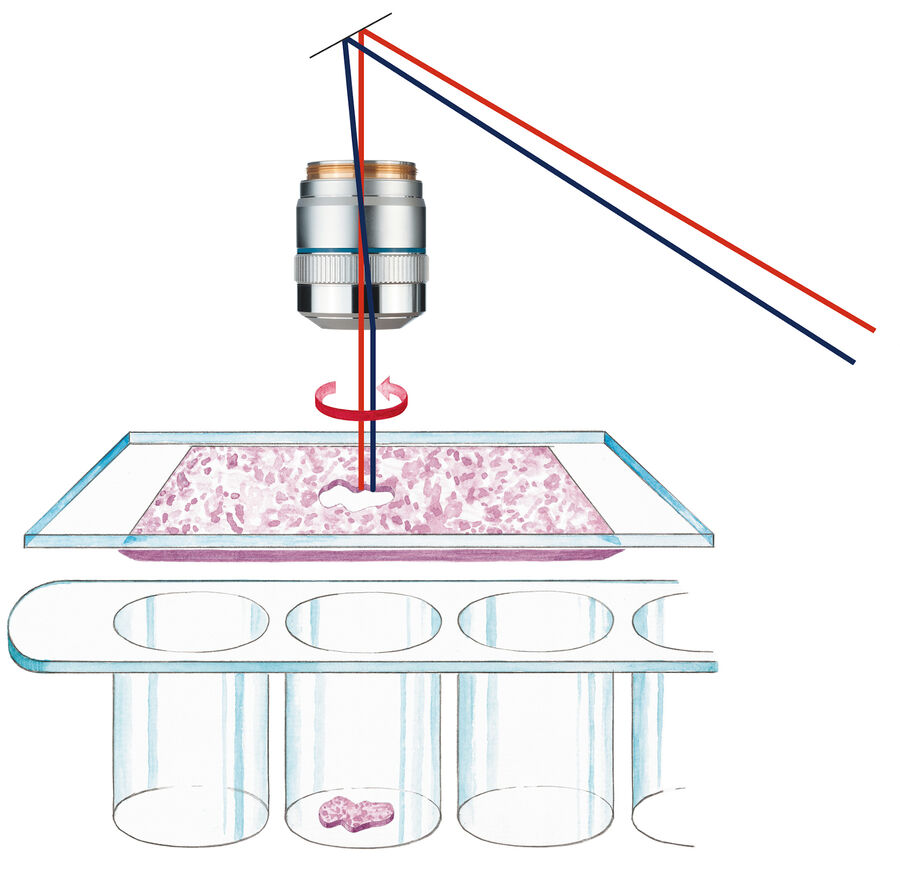
20 years ago, Leica Microsystems introduced the Leica AS LMD system. Meanwhile the PCR method was invented helping to deal with very little amounts of nucleic acids as template. In addition, pooling multiple dissectates into the same collection vessel simply by gravity allowed also to address mass spectrometry for proteomics or metabolomics by adequate pure starting material.
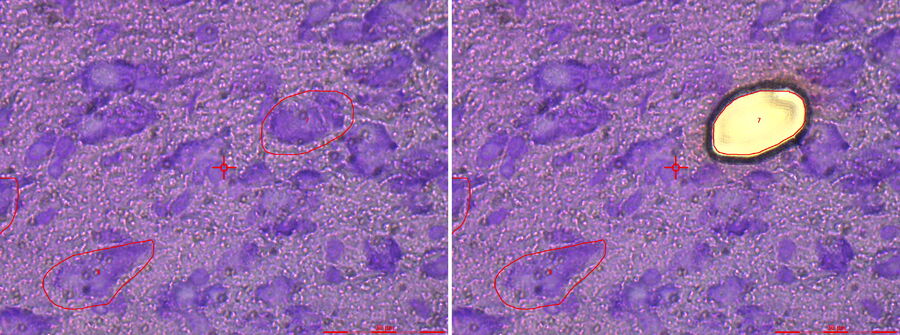
The Leica AS LMD is the foundation stone of success for meaningful science and diagnostics. Initially, laser microdissection was in particular interesting for cancer research and diagnostics. The power of isolating specific microscopic targets under visual control for downstream molecular biology analysis such as PCR or sequencing was and is extremely helpful for cancer diagnosis and investigations of tumors, metastasis and even sub-tumoural cells [3]. Diagnostic-wise it is critical to identify the exact underlaying mutation causing the tumor without any bias from surrounding healthy cells. This way specific treatments can be applied what is nowadays well-known under the term “personalized medicine” [3]. Beside cancer any other disease where affected cells differ in morphology from healthy cells can be target for purification under visual control by laser microdissection.
LMD Application Fields
In general, scientific-wise laser microdissection serves as a tool for region-, cell- and even chromosome-specific investigations and contributes to build up new knowledge and validate concepts lacking a specific proof.
Besides Cancer investigations laser microdissection conquered many different application areas. Neuroscience for example benefits from laser microdissection as this technology allows to separate different brain layers and even individual neurons [4,5]. The technique also contributed to the Allen Brain Atlas to validate in situ hybridization findings [6]. In Developmental Research laser microdissection allows to separate different layers in time-wise different developmental states [7]. With the addition of fluorescence even cell-cycle specific cells can be collected without contamination of cells in different cell cycle states than desired [8]. Live cell culture in general is a rare used, but beneficial application for laser microdissection, e.g. for cloning [9]. Dissection of plants is also an area for laser microdissection. The weight of plant material is beneficial for the gravity collection and the powerful laser from the LMD7 or the “laser screw” allows to dissect even thick material like native leaves [10,11].
Forensics is another discipline for laser microdissection. Most prominent example is the ability to separate sperm cells from an offender and skin cells of a victim allow here to bring cases to justice with definite evidence in from of DNA fingerprints of offender and victim [12,13].
Continuous Improvement and Innovation
The Leica LMD evolution: Since its introduction the Leica LMD systems evolved along customer needs and expectations to serve as a reliable research tool to link microscopy with molecular biology.
Highlights are:
2001 – Launch Leica AS LMD & introduction dedicated fluorescence module
2002 – Introduction of a database for automated image storage
2003 – Introduction special 150x dry objective for LMD & specimen overview
2005 – Launch LMD6000, LMD scanning stage & pattern recognition module
2007 – Draw+Scan mode for membrane free collection
2009 – Launch LMD6500 & LMD7000
2010 – Introduction Lab on a Chip collection device (nowadays: universal holder)
2011 – Dedicated LMD camera CC7000
2012 – special 10x objective for high laser power application & Laser Screw
2013 – 48-well collector, 3rd party pattern recognition access, Point-to-Point dissection for large areas
2014 – 1000th Leica LMD system sold
2015 – Launch LMD6 & LMD7, Shadow cuts
2016 – Database reporting function
2017 – Launch LMT350 for direct collection into 96-well plates, special 2.5x LMD objective
2018 – Final pulse cutting mode
2019-2021 – 384-well plate collection option, shape import & throughput optimization
Within the last 20 years not only more and more applications for the Leica LMD systems occurred. The system was also continuously improved to serve user needs and demands. This approach of step-wise improvement in close dialog with customers to fully understand demands on the system made the Leica LMD system the ideal tool for today’s ambitious workflows, especially for high-throughput applications and combinations with latest developments such as AI [14]. Cancer biomarker research is the main target for laser microdissection [15]. Maybe Leica LMD systems can contribute to future cancer vaccines. BioNtech, who invented the first COVID-19 vaccine, uses a laser microdissection system for their discoveries at least in their traditional cancer research branch [16].
Current State
Dr. Falk Schlaudraff, former Product Manager Leica Laser Microdissection Systems and meanwhile Manager Application Management Research Microscopy & Sample Preparation in the Business Unit Life Science remembers that he personally installed and trained the Leica LMD7000 at BioNtech headquarters in Mainz about 5 years ago: “Seeing this great technology applied by such high ranked and skilled researcher as from BioNtech makes me proud. Biomarker research approaches for cancer and COVID-19 are quite similar. This past success of Laser microdissection in the last 20 years makes me sure that along with BioNtech researchers from WHIRC around Thomas Conrads or the Novo Nordisk Foundation around Matthias Mann will improve future healthcare with the aid of our Leica LMD systems. I am grateful for the past years where I had the pleasure to travel and meet researcher around the globe and discuss about the LMD technology, applications and also to build up fruitful networks and friendships. Hopefully the days of global travel come back in the near future. Luckily, we have the option to connect via the internet with video chats etc. However, personal social interaction cannot be replaced in a digital way. To get this comfort of personal social interactions motivates me enable researchers around the world to find cures, vaccines, biomarkers and solutions for todays and future problems with the aid or our systems."
References
- Isenberg G, Rathke PC, Hülsmann N, Franke WW, Wohlfarth-Bottermann KE: Cytoplasmic actomyosin fibrils in tissue culture cells: direct proof of contractility by visualization of ATP-induced contraction in fibrils isolated by laser micro-beam dissection. Cell Tissue Res. 1976 Feb 27;166(4):427-43. doi: 10.1007/BF00225909.
- Isenberg G, Bielser W, Meier-Ruge W, Remy E: Cell surgery by laser micro-dissection: a preparative method. J Microsc. 1976 May;107(1):19-24. doi: 10.1111/j.1365-2818.1976.tb02419.x.
- Kölble K: The LEICA microdissection system: design and applications. J Mol Med (Berl). 2000;78(7):B24-5.
- Burbach GJ, Dehn D, Del Turco D, Deller T: Quantification of layer-specific gene expression in the hippocampus: effective use of laser microdissection in combination with quantitative RT-PCR. J Neurosci Methods. 2003 Dec 30;131(1-2):83-91. doi: 10.1016/s0165-0270(03)00232-2.
- Elstner M, Morris CM, Heim K, Bender A, Mehta D, Jaros E, Klopstock T, Meitinger T, Turnbull DM, Prokisch H: Expression analysis of dopaminergic neurons in Parkinson's disease and aging links transcriptional dysregulation of energy metabolism to cell death. Acta Neuropathol. 2011 Jul;122(1):75-86. doi: 10.1007/s00401-011-0828-9. Epub 2011 May 4.
- Jones A: A map of the brain. TEDGlobal 2011
- Ikeda K, Mutoh M, Teraoka N, Nakanishi H, Wakabayashi K, Taguchi R: Increase of oxidant-related triglycerides and phosphatidylcholines in serum and small intestinal mucosa during development of intestinal polyp formation in Min mice. Cancer Sci. 2011 Jan;102(1):79-87. doi: 10.1111/j.1349-7006.2010.01754.x.
- Handschick K, Beuerlein K, Jurida L, Bartkuhn M, Müller H, Soelch J, Weber A, Dittrich-Breiholz O, Schneider H, Scharfe M, Jarek M, Stellzig J, Schmitz ML, Kracht M: Cyclin-dependent kinase 6 is a chromatin-bound cofactor for NF-κB-dependent gene expression. Mol Cell. 2014 Jan 23;53(2):193-208. doi: 10.1016/j.molcel.2013.12.002.
- Podgorny OV: Live cell isolation by laser microdissection with gravity transfer. J Biomed Opt. 2013 May;18(5):55002. doi: 10.1117/1.JBO.18.5.055002.
- Hölscher D, Shroff R, Knop K, Gottschaldt M, Crecelius A, Schneider B, Heckel DG, Schubert US, Svatos A: Matrix-free UV-laser desorption/ionization (LDI) mass spectrometric imaging at the single-cell level: distribution of secondary metabolites of Arabidopsis thaliana and Hypericum species. Plant J. 2009 Dec;60(5):907-18. doi: 10.1111/j.1365-313X.2009.04012.x.
- Pouzoulet J, Scudiero E, Schiavon M, Rolshausen PE: Xylem Vessel Diameter Affects the Compartmentalization of the Vascular Pathogen Phaeomoniella chlamydospora in Grapevine. Front Plant Sci. 2017 Aug 21;8:1442. doi: 10.3389/fpls.2017.01442.
- Di Martino D, Giuffrè G, Staiti N, Simone G, Tuccari G, Saravo L: LMD as a forensic tool in a sexual assault casework: LCN DNA typing to identify the responsible. International Congress Series, Volume 1288, April 2006, Pages 571-573
- Leonov S, Zemskova E, Ivanov P: LMD-assisted single cell DNA typing of forensic biological evidence: Issues of the cell type and sample condition. Forensic Science International: Genetics Supplement Series, Volume 3, Issue 1, December 2011, Pages e47-e48
- Mund A, Coscia F, Hollandi R, Kovács F, Kriston A, Brunner A-D, Bzorek M, Naimy S, Rahbek Gjerdrum LM, Dyring-Andersen B, Bulkescher J, Lukas C, Gnann C, Lundberg E, Horvath P, Mann M: AI-driven Deep Visual Proteomics defines cell identity and heterogeneity. In preparation
- Bateman NW, Teng PN, Hope E, Hood BL, Oliver J, Ao W, Zhou M, Wang G, Tommarello D, Wilson K, Litzy T, Conrads KA, Hamilton CA, Darcy KM, Casablanca Y, Maxwell GL, Bae-Jump V, Conrads TP: Jupiter microtubule-associated homolog 1 (JPT1): A predictive and pharmacodynamic biomarker of metformin response in endometrial cancers. Cancer Med. 2020 Feb;9(3):1092-1103. doi: 10.1002/cam4.2729.
- Hartmann K, Schlombs K, Laible M, Gürtler C, Schmidt M, Sahin U, Lehr HA: Robustness of biomarker determination in breast cancer by RT-qPCR: impact of tumor cell content, DCIS and non-neoplastic breast tissue. Diagn Pathol. 2018 Oct 20;13(1):83. doi: 10.1186/s13000-018-0760-6.