Introduction
The optic nerve, which establishes contact between the retina and the brain, is made up of retinal ganglion cell (RGC) axons. These axons span a relatively large distance and function to sustain the visual process. In mammals, such as humans, the optic nerve possesses a severely limited capacity for self-repair following injury, often resulting in complete vision loss. To improve outcomes for these patients, we first need to fully understand the mechanisms employed in naturally regenerative species. In the frog, damage to the optic nerve results in spontaneous, full regeneration and restoration of vision. This provides us with an ideal model system to understand underlying mechanisms involved in successful optic nerve regeneration and to identify targetable pathways for eventual vision-preserving therapies in regenerative-incompetent species.
Challenges in manual optic nerve injury models
Manual surgical manipulation of the tadpole optic nerve using a dissecting microscope requires advanced microsurgical skill and is challenged by the small size and fragility of the animal. Even with micromanipulators and fine forceps, manual transections are invasive, difficult to standardize across operators, and often result in high mortality rates. Lesion size and placement can vary considerably, introducing inconsistency across experimental replicates. These technical limitations compromise the reproducibility of the model and reduce the statistical power of downstream analyses. As a result, drawing reliable conclusions becomes difficult, especially in studies requiring precise spatial or temporal control of optic nerve injury.
Novel Laser Microdissection method for optic nerve injury
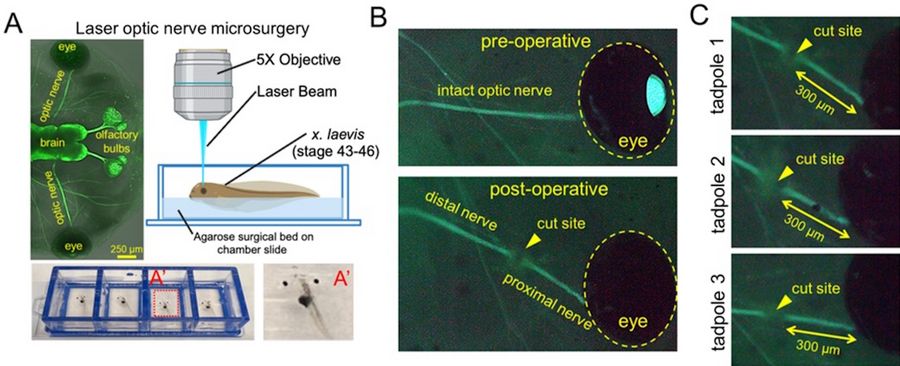
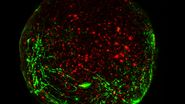
To develop a new surgical model, LMD7 was utilised alongside the Xla.Tg (tubb2b: mapt-GFP)Amaya transgenic X. laevis line to visualize and perform optic nerve transection and subsequent axonal regrowth. X. laevis tadpoles were used at developmental stages 43–46. At these stages, the tadpoles are naturally transparent and can be used in live-fluorescent imaging. Briefly, tadpoles were anesthetized and placed onto custom surgical beds made of 4% agarose inside a chamber slide. Tadpoles were oriented so they were flat with their dorsal side up. The left optic nerve of each tadpole was transected with a 349 nm UV cutting laser at 5× magnification, ~300 μm distal to the exit of the optic nerve from the globe (using the “measure” tool in the Leica LMD7 software). The line drawn across the optic nerve with the “draw” tool received four laser pulses (120 µJ/pulse) of 55 mW laser (pulse frequency = 2710), on laser screw mode (step count = 2, step size = 5 μm, repeats = 2) to cut to the appropriate depth and transect the nerve. The cut was confirmed by a gap in the fluorescent optic nerve visualization. Tadpoles were then immediately removed, recovered in 1X MMR media, and used in later downstream experiments.
Results
This laser microdissection method enables high-throughput, reproducible, and minimally invasive optic nerve transections, resulting in robust retinal ganglion cell (RGC) axon regeneration within a short timeframe following injury. Leveraging the precision of LMD7 ensures consistent lesion placement across biological replicates, which is critical for downstream analyses. This model has been applied to study axon dynamics through longitudinal confocal imaging, map the timeline of structural degeneration and regeneration (over ~14 days), investigate cell death and proliferation, assess visual pathway recovery, and characterize early transcriptomic responses via RNA-seq. Additionally, the protocol is compatible with other transgenic tadpole lines, such as those expressing genetically encoded sensors. For example, the Xla.Tg (tubb2b:GCaMP6s)NXR line was used to visualize calcium dynamics in the optic nerve at the onset of laser injury.
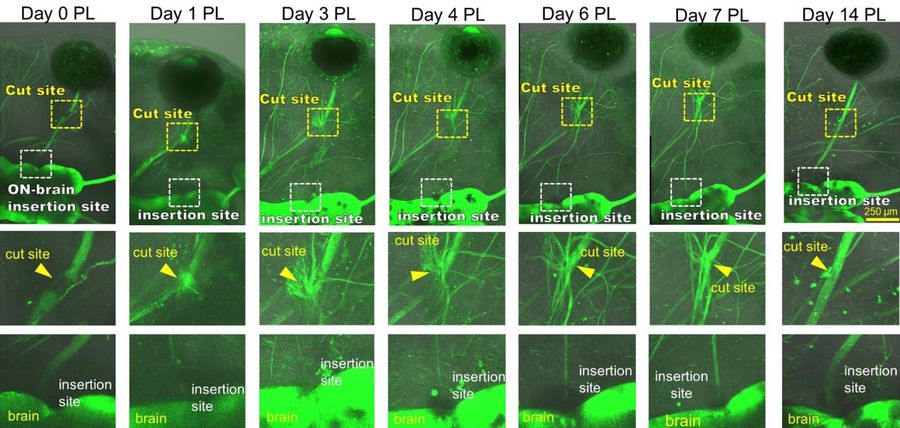
Axons distal to the injury site degenerate from day 0–2 post laser (PL), followed be several axons sprouting from the proximal cut end in all directions from the injury site by day 3 PL. Some regrowing axons quickly reach the brain after which this connection becomes reinforced by additional axons that follow the path of those pioneer axons. After 14 days, the optic nerve is virtually indistinguishable from the naïve (uninjured) optic nerve morphology.
Video 1: Three days post-transection, axonal sprouting is observed emerging from the injury site of the left optic nerve, while the contralateral nerve remains intact. Z-stack images were acquired on a Leica confocal microscope from anesthetized Xenopus laevis tadpoles, allowing visualization of regeneration dynamics in situ.
Video 2: Calcium dynamics are visualized with the Xla.Tg (tubb2b:GCaMP6s)NXR line. Imaging was performed continuously during and after laser transection. Laser injury induces rapid calcium entry into the nerve. Video is sped up and fluorescence intensity is shown as a heatmap.
Conclusion
This LMD laser-based approach provides the precision needed to generate consistent lesions with minimal off-target damage, overcoming the limitations of manual techniques. As a result, it offers a powerful and reproducible platform for studying mechanisms of CNS regeneration. This model can be used for future experiments involving targeted gene manipulation, expanding the toolkit for regenerative neuroscience research.