Early detection and molecular profiling of disease progression remain major challenges in modern medicine. Despite advances in genomics and transcriptomics, spatial proteomic mapping of tissue lesions is still largely unexplored. This approach provides unprecedented insight into the molecular landscape of disease progression, supporting the discovery of novel biomarkers and therapeutic targets.
One highlight was the application in toxic epidermal necrolysis (TEN), a life-threatening skin reaction. DVP identified STAT1 as a key driver, leading to the successful use of a JAK inhibitor—eight out of eight patients were cured [1].
Another example focused on low-grade serous ovarian cancer. By combining DVP with spatial transcriptomics, two target proteins (FOLR1 and CDK2A) were identified, for which clinically approved antibodies already exist. Mouse models showed a significant reduction in tumor burden.
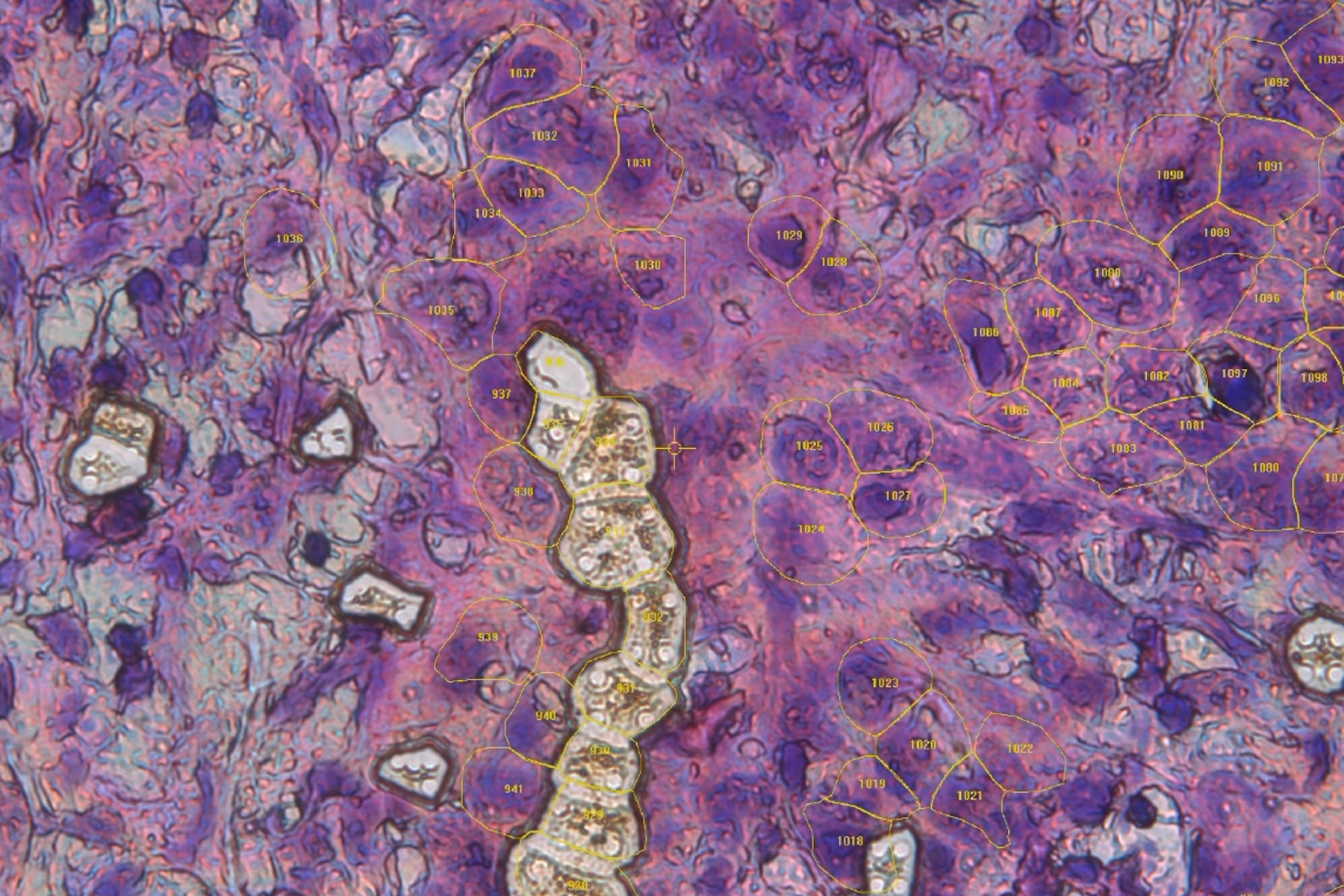
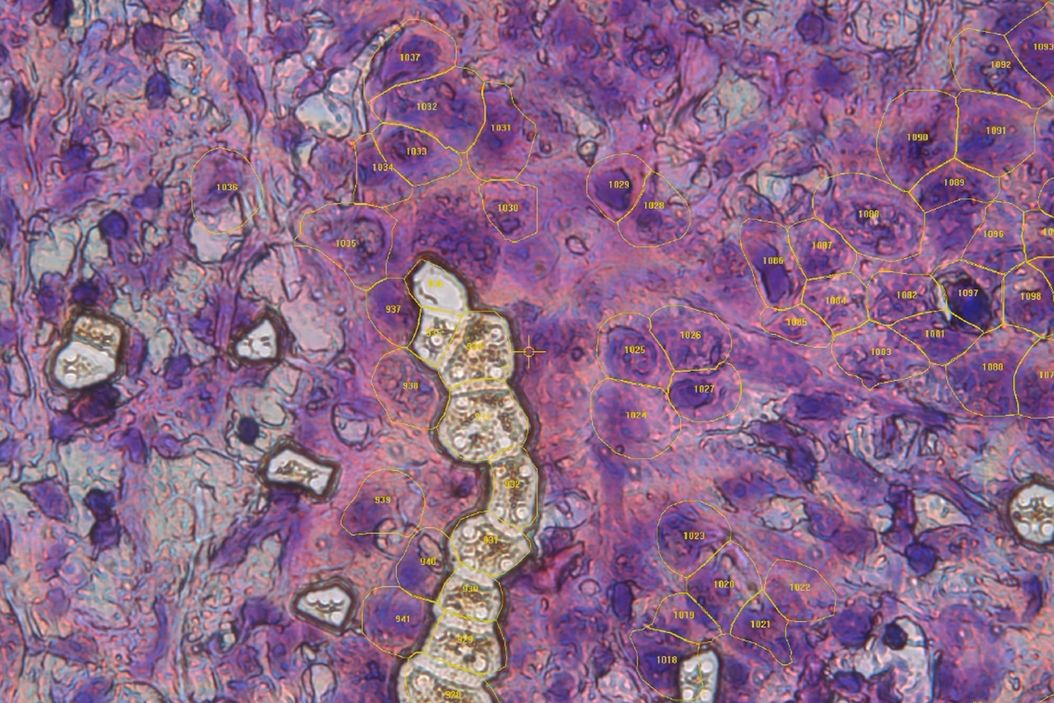
Finally, Dr. Mund demonstrates DVP’s use in pancreatic cancer research. AI-guided image analysis identified early precursor lesions, which were then proteomically characterized. This opens new possibilities for early detection and targeted therapy development.
This webinar is a must-view for researchers aiming to advance early disease detection, decode cellular mechanisms, and leverage AI-driven spatial proteomics for breakthrough insights in pathology and personalized medicine.