You can learn how to:
- Overcome the challenges of staining and imaging injured cardiac tissue
- Visualize and differentiate injured and proliferating cardiomyocytes
- Optimize the imaging and reconstruction of organ explants
Speakers

Laura Peces-Barba Castaño
PhD student - Prof. Dr. Stainier Department, Max Planck Institute for Heart and Lung Research
Laura holds a degree in Biomedical Sciences at the University of Aberdeen (Scotland). During this time, she also did an Industrial Placement at the Francis Crick Institute working with malaria parasites. Currently, Laura is undertaking a PhD in heart regeneration in the Stainier Department (Max Planck Institute), where she aims to discover the mechanisms leading to heart regeneration in the zebrafish.

Dr. Lynne Turnbull, Senior Application Manager – Leica Microsystems
Lynne is a Senior Application Manager at Leica Microsystems. She received her PhD in Sydney Australia in cardiac biophysics and undertook postdoctoral training in San Francisco and Melbourne. Upon moving to the University of Technology Sydney, Lynne established and managed the Microbial Imaging Facility. Lynne joined Leica Microsystems in 2021 as a Senior Application Manager and is based at the EMBL IC in Heidelberg.
The Experiment
Original broadcast date: 25th May 2022
In this video we talked with our guest, Laura Peces-Barba Castaño, about how hearts regenerate after injury in the model organism zebrafish. Mammalian hearts have a very limited capacity to regenerate after injury, leading to the presence of scar and fibrotic tissue. However, zebrafish hearts can regenerate after severe injury without scar formation, making the zebrafish a great experimental tool to explore cardiac injury.
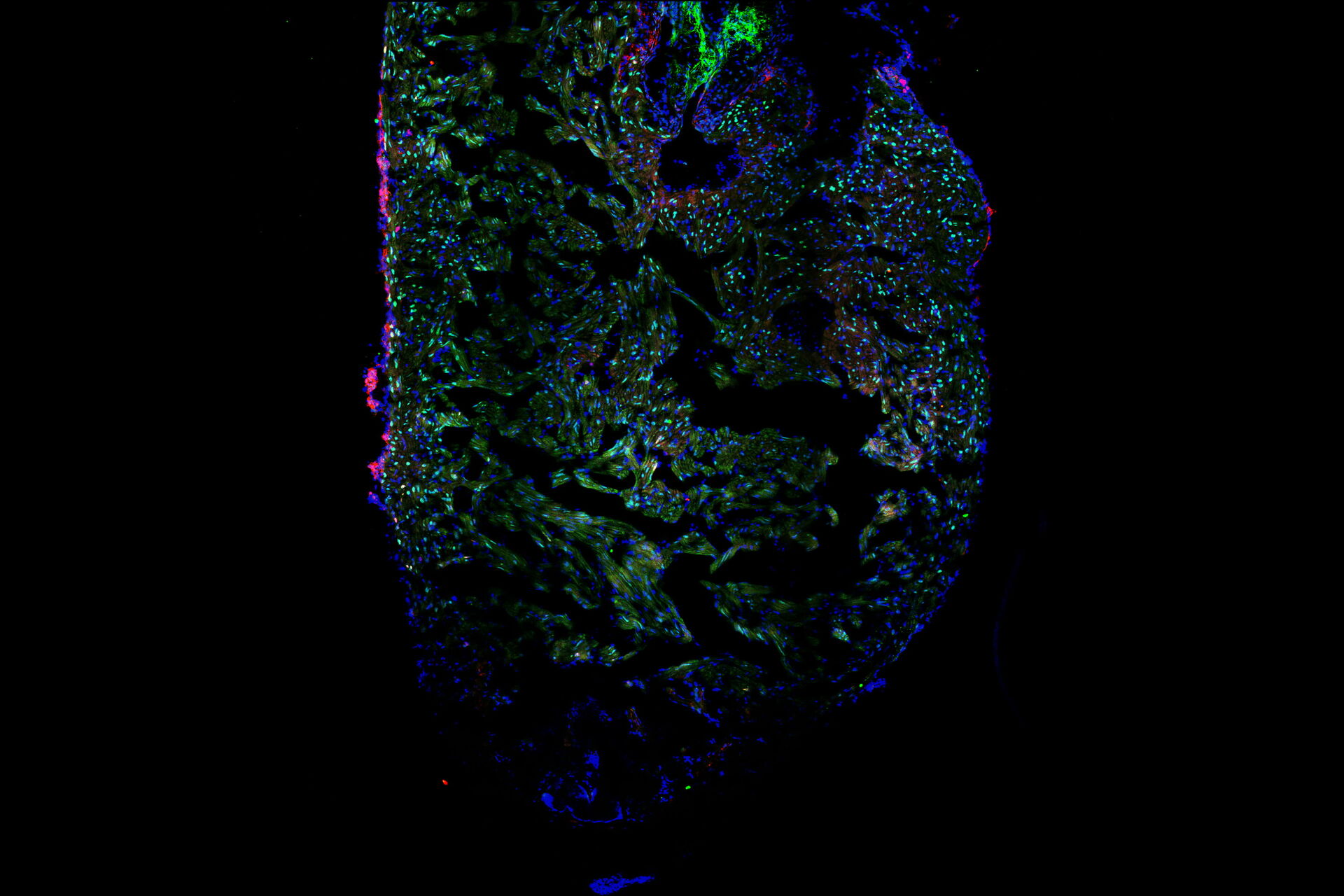
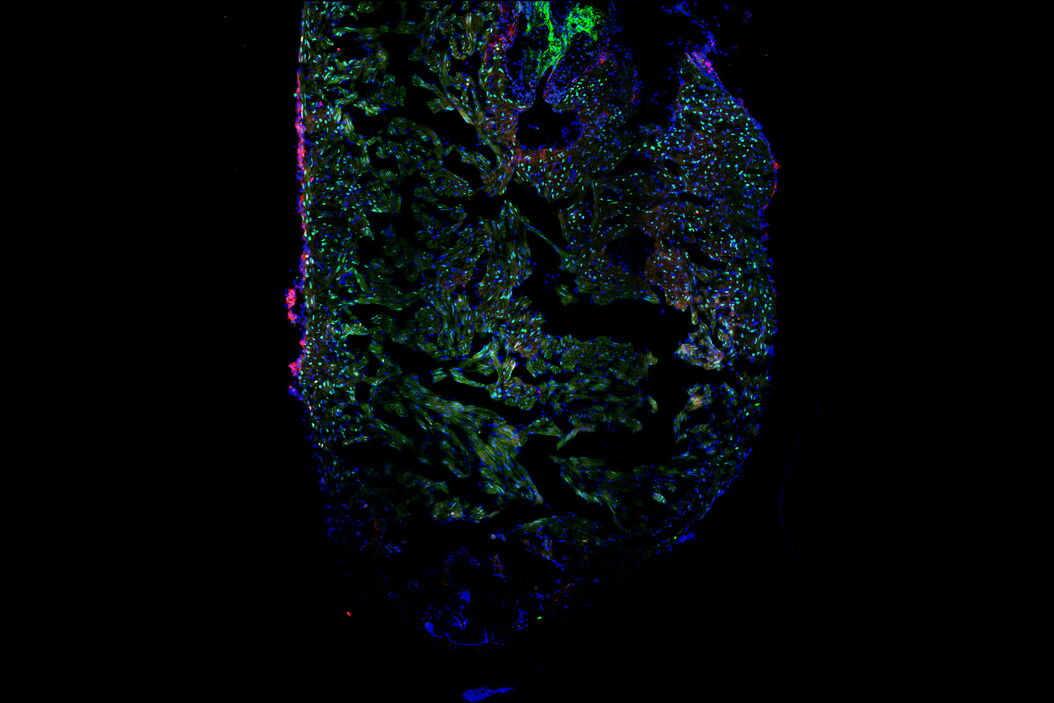
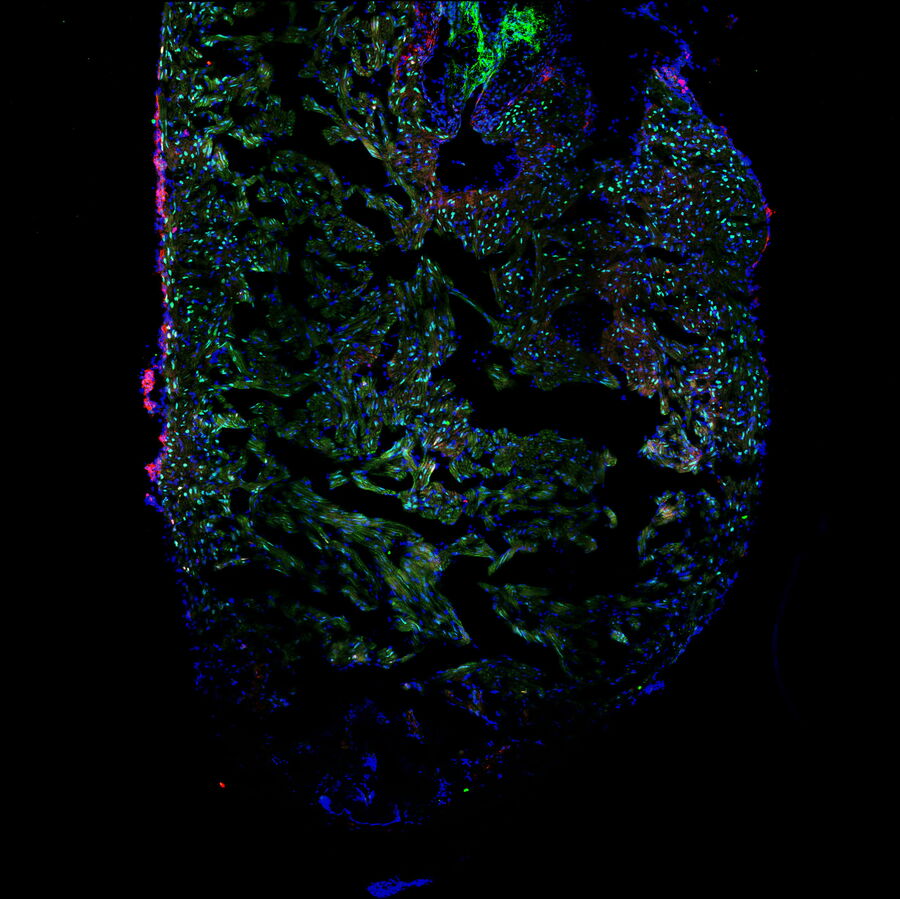
Laura showed us the experimental procedure to injure the tip of a zebrafish heart and how she processed the hearts for imaging. We learned to identify the border zone between the injured and non-injured tissue and how this zone is the most interesting area for studying heart recovery. She used specific staining of cardiomyocytes, proliferating cells, and all cells to create differential staining patterns within the border zone. The differential staining patterns allowed the quantification of injured and proliferating cardiomyocytes. Laura also described some of the challenges of when processing, staining, and imaging the cardiac tissue sections.
The ability of Mica’s sample finder to create a quick overview of the whole slide that contains 10 heart sections was a favorite feature for Laura, as it allowed easy navigation and exploration of the sections before deciding for which ones images were to be acquired. Tiled images of a single z-slice of each section could be captured rapidly in widefield and quickly examined. Then it was decided which sections are suitable for further observation with confocal imaging, either with or without z-stacks. This approach increased the efficiency of the workflow and reduced the time spent imaging. Laura was also very happy that all this could be done in a brightly lit room, instead of needing to be in a “dark cave” while doing microscopy!