Early fluorescence observations
The interest of man in fluorescent proteins can be traced back to the first century A.D. when the roman natural philosopher Pliny the Elder [1] (Gaius Plinius Secundus, 23 A.D. – 79 A.D.) described a glowing jellyfish (Pulmo marinus) in the Mediterranean Sea. In his eyes these animals emitted light of such intensity that it could almost be used as a torch (Bohn, 1855). Beside this jellyfish there is an abundance of other organisms attracting our attention because they glow in the dark. In fact they don’t do this to catch our admiration but, amongst other theories, to communicate with their conspecifics (fireflies exert a pull on their mates e.g.), attract prey (frogfish e.g.) or to frighten predators (bobtail squid e.g.). Some of these fascinating creatures are living in the world of darkness – such as the deep sea – where light is a raw property. Whereas only the upper 200 m of the oceans are penetrated by sunlight, underneath that border the only existing illumination is produced by organisms which are living there.
Using chemiluminescence and fluorescence to emit light, evolution developed one of the most impressive and powerful tools of recent biochemistry and cell biology which is based on the scientific curiosity of several persons. Their efforts and discoveries should be mentioned in the following.
With the upcoming interest in natural sciences after the Middle Ages, people like the Spanish physician and botanist Nicolás Monardes described a gleaming wood extract made of Lignum nephriticum in 1565. Later on this light could be ascribed to an oxidation product of a flavonoid which is found in this plant and showed that glowing was not only restricted to animals. More than three centuries later (1858) George Gabriel Stokes, an Irish mathematician and physicist, formed a name for that kind of natural occurring phenomena: Fluorescence. He thought up this term after the blue light emitting mineral fluorite and broadened the observation of radiating objects from animals over plants to stones.
The breakthrough for GFP
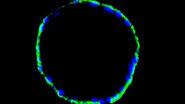
One of the first researchers in modern times, who started the systematical exploration of fluorescent proteins, was the Japanese biochemist Osamu Shimomura. He shared the interest of Pliny the Elder in fluorescent jellyfish and established Aequorea victoria as his "object of desire" in the early 1960’s. Shimomura was fascinated by A. victoria because it is able to emit green light by means of fluorescence. Its habitat is in the Pacific Ocean were it feeds on shellfish and other jellyfish. The light is produced in the so called luminescent organs arranged in a ring on the underside of the organism. Shimomura, who did studies about luciferin of some seed shrimp during his graduation already, dissected these organs and squeezed them through a sieve with the result of a faint luminescent slurry. By doing so with thousands of jellyfish Shimomura’s effort was rewarded by the extraction and purification of the chemiluminescent protein aequorin [2]. Later on it was discovered that aequorin was responsible for the emission of blue light which was due to its role as a luciferase in the oxidation of coelenterazine in a calcium-dependent reaction. Luciferases are enzymes which are oxidizing their substrates (Luciferines) in a chemical reaction, associated with the emission of light.
Nevertheless there was this puzzling question why there was blue light emitted in the extraction while the intact organism glowed in green? It took Shimomura and colleagues several years to solve this mystery. But finally their studies revealed that the blue light coming from aequorin, was only the source of energy for another gleaming protein – the jellyfish green fluorescent protein (GFP). They realized that the light emitted by aequorin was absorbed by GFP which used this energy to produce a green fluorescence.
The next path-breaking step in the triumphal procession of GFP had to wait for the invention of cloning techniques in the 1970s. It was the work of the American microbiologist Douglas Prasher who cloned the complete GFP gene for the first time in 1992 [3]. Before he prepared an A. victoria cDNA library together with his colleague Milton Cormier at the University of Georgia, knowing already the prospective of GFP. Unfortunately Prashers funding by the American Cancer Society ran out before he could start to express the recombinant GFP in bacteria.
Initial doubts that GFP would not work outside jellyfishes were erased by Martin Chalfie, an American biologist who could benefit from Prasher’s preliminary work. By introducing the GFP gene into E. coli and the nematode C. elegans he could show that there were no other jellyfish specific proteins or factors necessary to produce the green glow [4].
GFP in live cell imaging
With that experience the door was opened for the green fluorescent protein to start its astonishing career in life sciences. GFP became the key to observe structures in living cells and organisms without the necessity of synthetic labels or fluorescent antibodies. In the case of immunofluorescence staining, the access of antibodies to accordant target structures inside the cell has to be guaranteed. This is achieved by permeabilizing the cells with detergents (e.g.), which inevitably induces cell death. Furthermore most antibodies have a preference for denatured antigens. Therefore immunofluorescence techniques use agents like para formaldehyde to denature cellular protein target structures. Altogether the use of GFP could overcome these non physiological conditions and paved the way for life cell imaging.
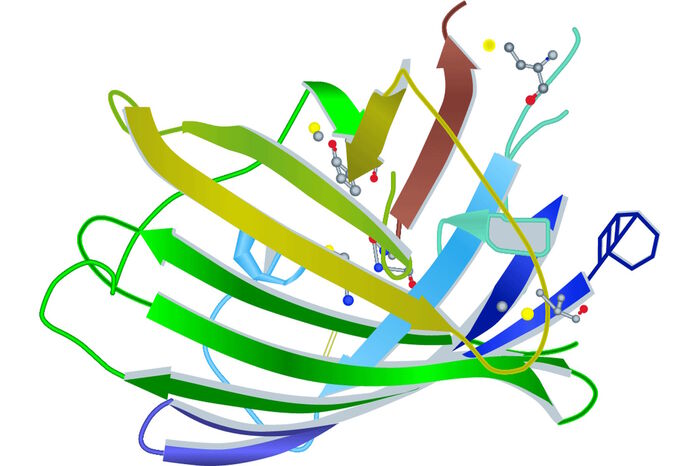
Another person who was realizing and developing the great potential of GFP was Roger Tsien. Coming from the Calcium regulation field, the American cell biologist was getting interested in tracking macromolecular interactions inside living cells. After studying and working in Harvard, Cambridge and Berkeley he finally settled down at the University of California, San Diego. As a Professor of Pharmacology, Chemistry and Biochemistry he improved the efficiency of GFP by taking advantage of a very common principle in evolution: Mutation. In 1994, he and his group established a single point mutant (S65T) of GFP with a much better intensity and photo stability than the wild type version. Besides a brighter glow the S65T mutant had another striking technical benefit. Whereas the wild type GFP had two excitation maxima at 395 nm and 475 nm, the mutated version had only one at 484 nm. By keeping its emission wavelength at 509 nm, the spectral characteristics of the "new" GFP fitted almost into classical FITC fluorescence properties (FITCex: 496 nm, FITCem: 520–530 nm). Because of its enhancements this GFP variant was called “enhanced” GFP or EGFP.
By doing structural research on GFP Tsien and coworkers developed further fluorescent derivates. With the GFP structure in their hands they established a variant (T203Y) which was shining in bright yellow and was therefore given the name "yellow fluorescent protein" or YFP. Cyan (CFP) and blue (BFP) forms followed [5].
Fluorescent Proteins in Anthozoa
The colour palette of fluorescent proteins was extended into the red range of the spectrum when the biochemist Sergey A. Lukyanov, who is a corresponding member of the Russian Academy of Sciences since 2003, discovered red fluorescing proteins in corals [7]. After buying some of these Cnidaria in a moscow pet shop Lukyanov studied the fluorescent behavior of these primitive animals destined for russian marine aquariums. Amongst others he identified a red fluorescing protein coming from Discosoma – a Corallimorpharia – which he called DsRed. Besides DsRed, Lukyanov and coworkers identified a whole palette of other glowing proteins in Anthozoa, which were optimized for biochemical use time after time.
The Nobel Prize
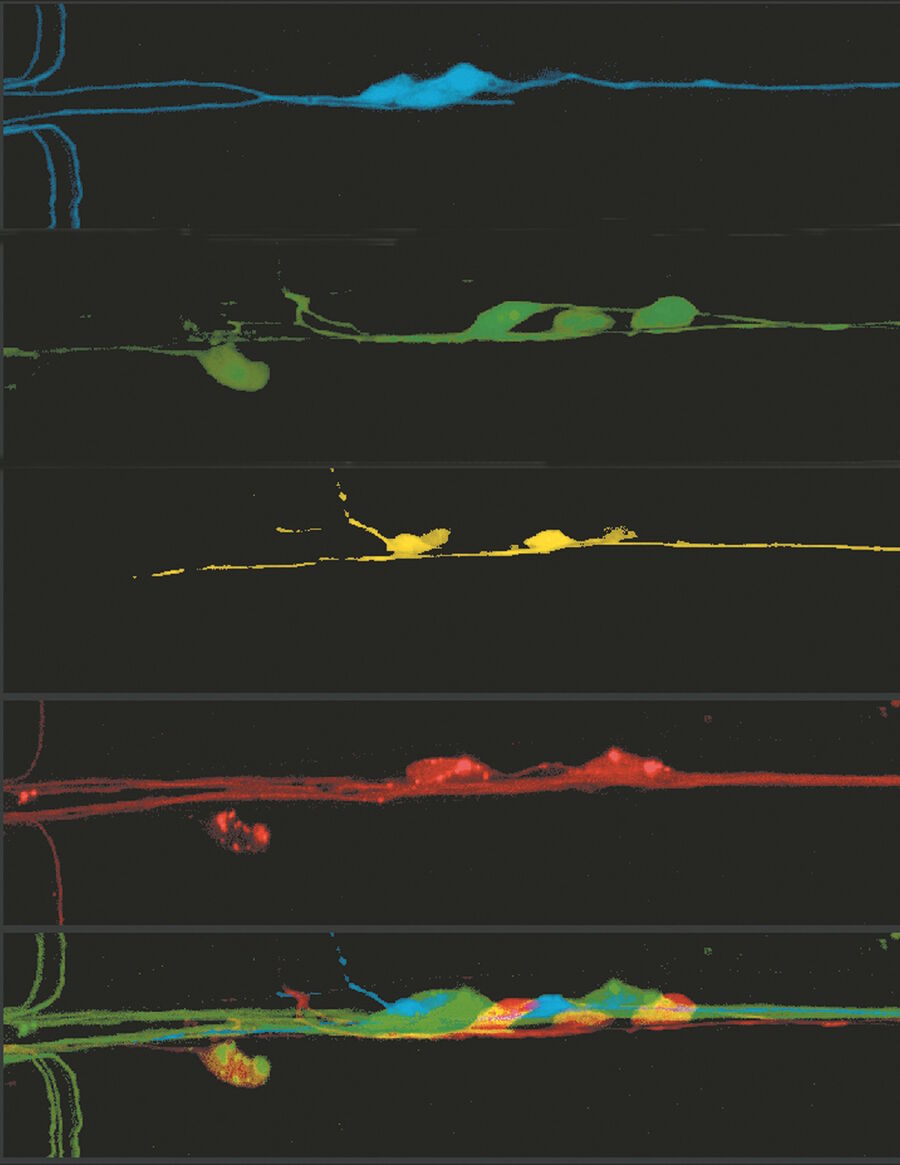
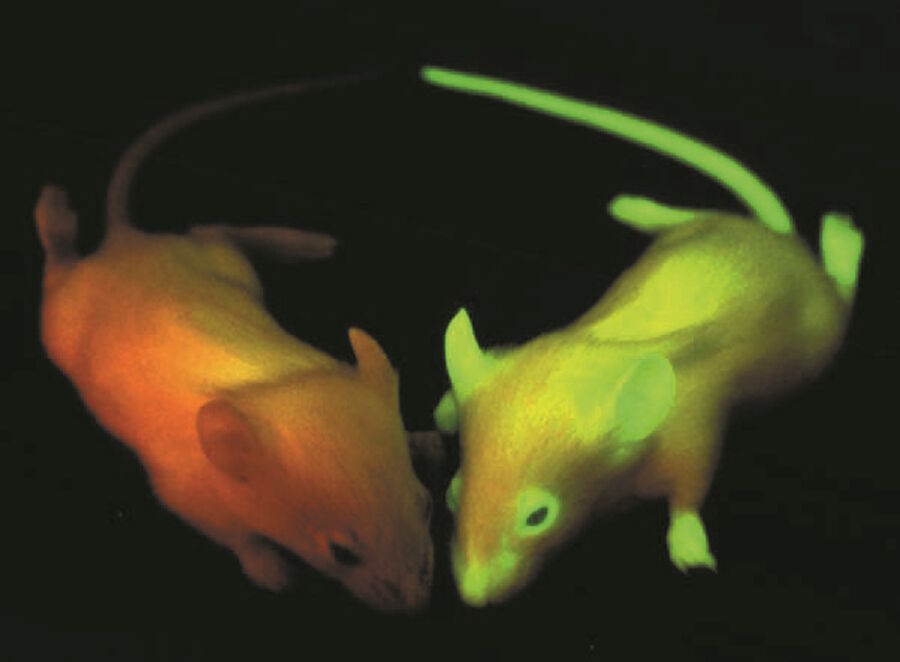
All the effort researches put into the discovery, understanding and enhancement of fluorescing proteins lead to a huge range of applications in life sciences. GFP and its variants opened the door for scientists to watch f.e. metastasis or angiogenesis in living organisms. Moreover the use of fluorescent multicolored neurons (Brainbow) will help to understand complex neuronal networks in the brain. With the possibility to decorate parasites like the Malaria pathogen Plasmodium falciparum with fluorescent proteins it is realizable to watch their fate in host cells. The list of opportunities could be continued almost endless and does not only include clinical relevant projects but also other basic science ventures.
All together the use of GFP and its mutants changed our view on life and its pathological modifications in a dramatic way, that three people who were involved in the discovery and development of GFP were rewarded by giving the Nobel Prize in Chemistry 2008 to them. Osamu Shimomura, Martin Chalfie and Roger Y. Tsien were decorated with the highest honors in science for their work on "the discovery and development of the green fluorescent protein, GFP".
www.nobelprize.org/nobel_prizes/chemistry/laureates/2008/
In the eyes of the Nobel Committee for Chemistry (2008), represented by Professor Måns Ehrenberg "… The discovery and development of the green fluorescent protein (GFP) have radically changed the scientific agenda. Improved variants of GFP and GFP-like proteins in synergy with high-resolution microscopes, computational techniques and powerful theoretical approaches are currently fuelling a scientific revolution focused on quantitative analyses of complex biological systems. The gradual appearance of a world of hitherto unseen structures and dynamic principles is now impacting virtually all aspects of biological, medical and pharmaceutical research …"
This quotation of the Presentation Speech for the 2008 Nobel Prize in Chemistry represents once more the high impact of fluorescent proteins on current life sciences and also arouses our curiosity on upcoming utilizations in the future. Recent developments, like highlighter proteins (photoactivateable, photoswitchable or photoconvertable FPs) and the establishment of new optical facilities like super-resolution microscopy make clear, that evolution of fluorescence is still in progress and far away from the end.
References
- Pliny JB and Riley HT: The natural history of Pliny, Book XXXII. Remedies derived from aquatic animals. Chapter 52 – Other aquatic productions. Adarca or Calamochnos: three remedies. Reeds: eight remedies. The ink of the sæpia. Gaius Plinius Secundus (Pliny the Elder). AD77. Bohn HG, London (1855).
- Shimomura O, Johnson FH and Saiga Y: J Cell Comp Physiol 59 (1962) 223–239.
- Prasher DC, Eckenrode VK, Ward WW, Prendergast FG and Cormier MJ: Gene 111 (1992) 229–233.
- Chalfie M, Tu Y, Euskirchen G, Ward WW and Prasher DC: Science 263 (1994) 802–805.
- Heim R, Prasher DC, Tsien RY: Wavelength mutations and posttranslational autoxidation of green fluorescent protein. Proc Natl Acad Sci USA 91 (1994) 12501–04.
- Ormo M, Cubitt AB, Kallio K, Gross LA, Tsien RY, Remington SJ: Crystal structure of the Aequorea victoria green fluorescent protein. Science 273 (1996) 1392–95.
- Matz MV, Fradkov AF, Labas YA, Savitisky AP, Zaraisky AG, Markelov ML, Lukyanov SA: Fluorescent proteins from nonbioluminescent Anthozoa species. Nature Biotech 17 (1999) 969–73.