Why is the constant control of oxygen important?
Oxygen is a fundamental factor for most life forms. For life science researchers, O2 is an electron acceptor, a highly reactive molecule that is essential for life and a molecule that affects every single event and reaction in biology. In general, the ambient oxygen ratio is considered to be 21%.
However, as air is inhaled tissue oxygenation drops to about 5%. In the liver to lung epithelium O2 levels are at about 14% and the lumen of intestinal mucosa is practically anaerobic. In between these percentages, there are various O2 levels in different tissues, but most importantly, there isn’t a single tissue where cells would face 21% of O2. In fact, 21% of O2 is highly toxic to cells due to the formation of reactive oxygen species (ROS) and their intermediates. When ROS production exceeds the cell’s antioxidant abilities, ROS begin to damage macromolecules, such as proteins, lipids, and DNA.
Physoxia is the level of O2 where cells and tissues can respond and maintain their preferred O2 level through increased blood flow, vasodilation, or upregulation of oxygen-responsive genes. These responses occur below a certain point that varies between tissues and cells. Normal tissues are maintained between 3% and 10% - a niche best described as physoxia – and levels below this are considered hypoxic conditions.
In research, many, or maybe even most, physiological phenotypes are hidden, because the studies are done under non-physiological conditions. Therefore, it is important to know the O2 level of the organ or tissue considered in the respective study to obtain translatable and biologically relevant experimental results. Each tissue and organ has its own oxygenation status which reflects its function. When either in too low or too high quantities, O2 has effects ranging from single gene expression to impacts on proteome level.
OxyGenie by Baker Ruskinn
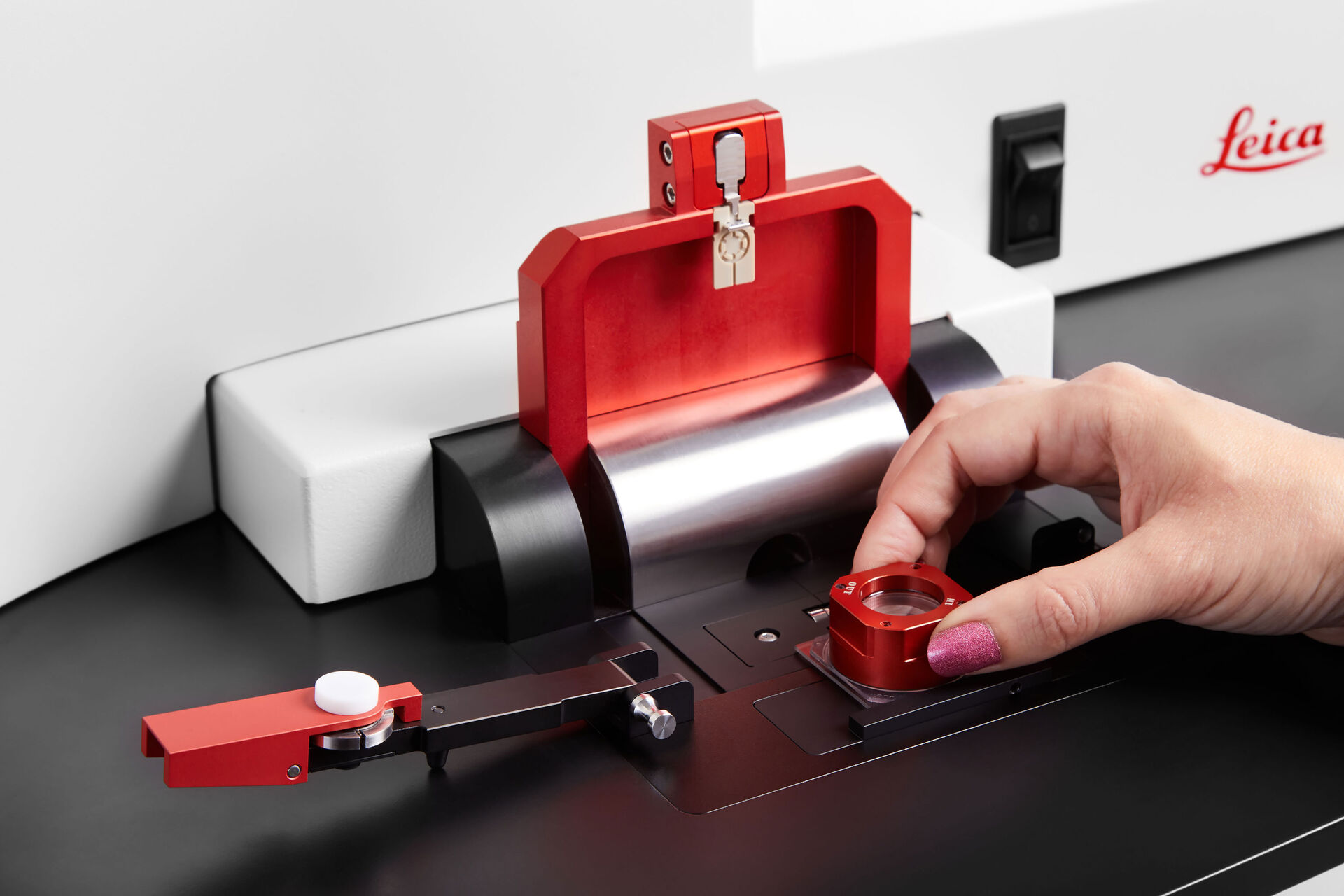
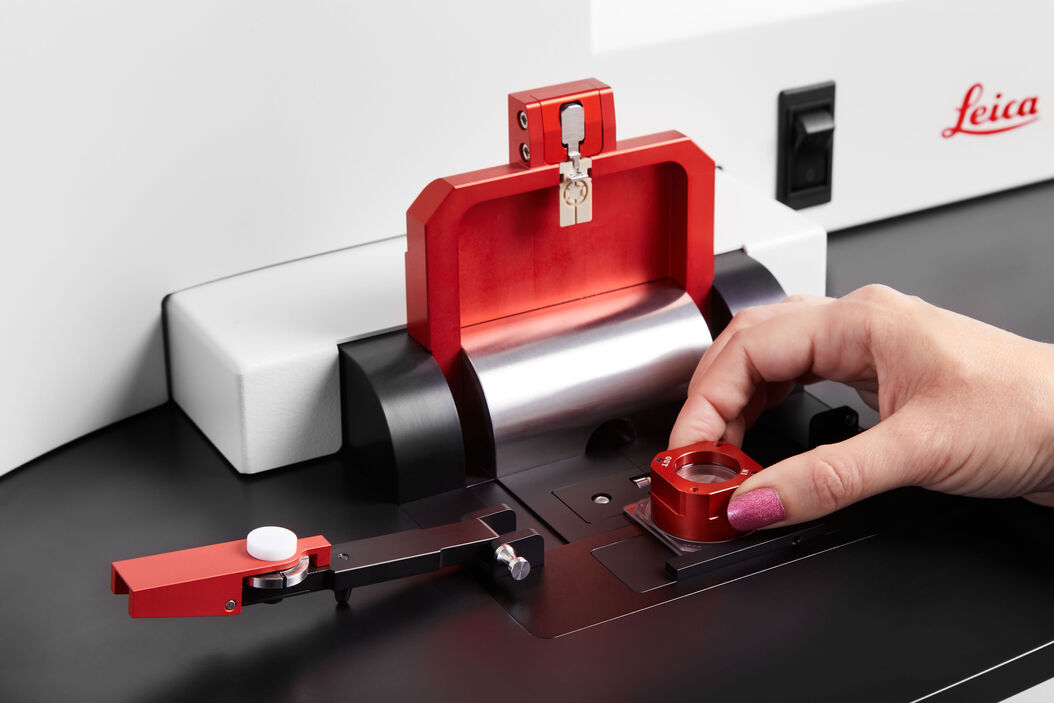
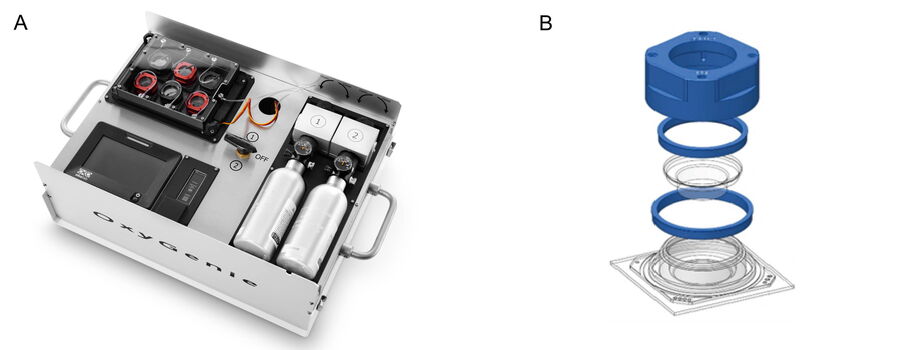
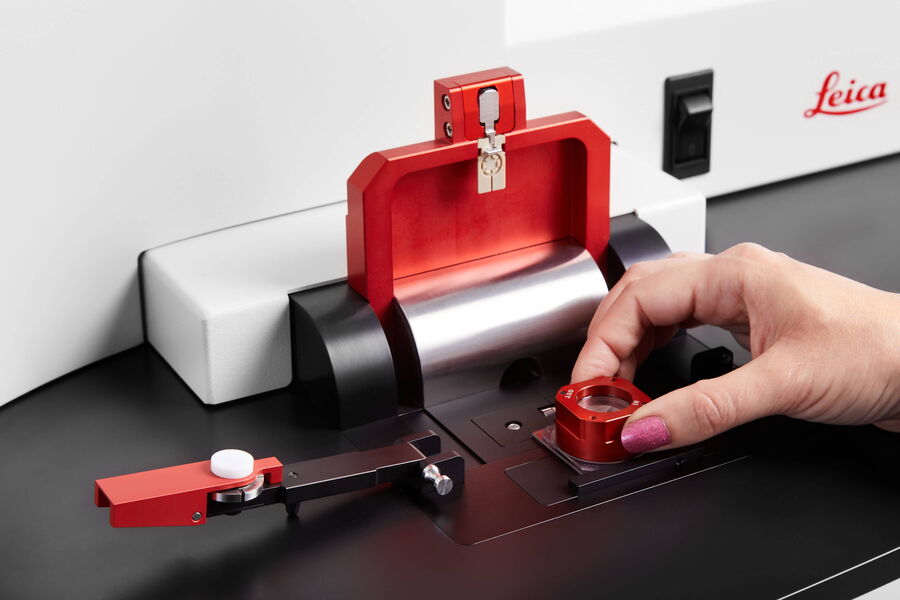
OxyGenie is a minimized, portable incubation platform keeping your samples under physoxia conditions or your desired atmosphere. The OxyGenie can be battery operated to control your samples not only in the lab, but wherever you go. It consists of 1 to 6 individual 1-well culture chambers, where each chamber is equipped with its own gas supply port. For gas control the OxyGenie has two refillable gas cylinders (Catalina Cylinders, CA, USA), that can be filled with your desired gas mixture. The gas flow is regulated by adjusting the outlet pressure from the cylinder. The operation time with two fully loaded gas cylinders for six sample chambers is about 16 h. The temperature is controlled by an ITO heater and controller (Okolab s.r.l., Italy). The individual sample chambers sit on a 6-well flow divider with which the cells can be separated from the base without interrupting physiological parameters, for example during light microscopy imaging.
The sample chambers are optimized to perform high-resolution imaging. High quality cover glasses are used as the base of the sample chambers. Different lid and locks transform each chamber into a mini incubator. The sample chamber is optimized for 1 ml of sample medium and sealed by an optical transparent glass lid. The lid lock prevents potential evaporation. The cover lock secures the cover airtight and firmly. The final cover provides the gas hose connections to the system.
Measurements on gas control of the samples over time: Metsälä et. al., Transportable system enabling multiple irradiation studies under simultaneous hypoxia in vitro (2018)
Adapted for the Coral Life
To maintain physoxia conditions throughout the complete live-cell CLEM workflow, the ability of the OxyGenie to ensure physiological conditions for samples until the moment of cryo fixation is taken advantage of. However, some changes were made in order to fit the needs of the workflow:
For high-pressure freezing on the EM ICE, 6 mm sapphire disks are used as sample substrates. Furthermore, to allow the best-possible live-imaging (see application note: How to Improve Live Cell Imaging with Coral Life), the possibility of immersion imaging on the sapphire discs is important. Therefore, the glass coverslips at the bottom of the chamber were exchanged with a 35 x 35 x 1 mm glass slide having a 6.5 mm hole in the center (referred to as the SampLink base cup). This hole is aligned with the EM ICE middle plate to have direct access to the center of the sapphire. Attachment of the middle plate and sapphire substrate to the bottom of the SampLink base cup is done with dental wax. The alignment and attachment of the middle plate on the SampLink base cup are described in section 5.2 of Coral Life operating manual.
After imaging, there must be a fast detachment of the EM ICE middle plate from the SampLink chamber. Therefore, the EM ICE loading area is equipped with a sliding and detachment mechanism which detaches the middle plate from the glass base and places it directly in the right position for freezing.
Cell viability and cytotoxicity in SampLink chambers
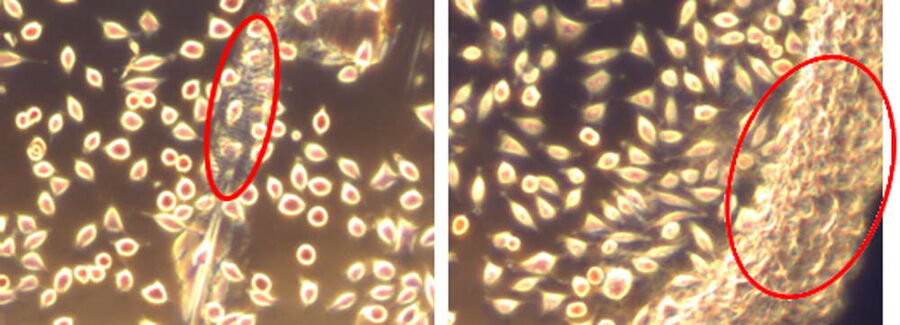
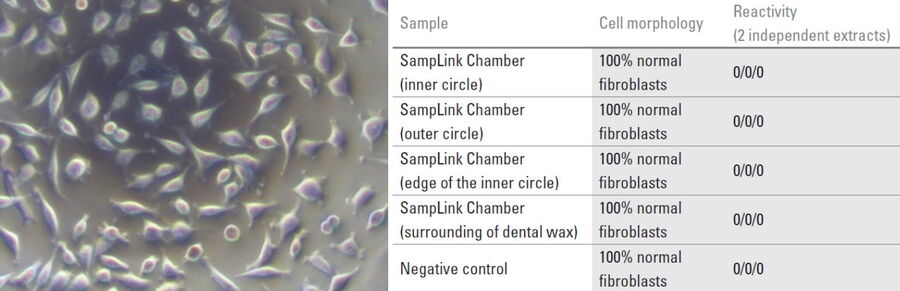
To ensure sample viability in the modified SampLink chambers, cell viability and cytotoxicity were tested. Tests were performed using the mammalian fibroblast cell line L-929 (ATCC Number CCL-1, Lot 13). Testing was executed by OFI Technologie & Innovations GmbH in Vienna, Austria. Cells (105 cells/ ml) were seeded either on the sapphire alone (50 μl) or on the whole sample chamber bottom (500 μl). For control, same cell dilutions were seeded in 96 well plates (50 μl) or 12 well plates (500 μl). Samples and controls were incubated for 24 h at 37° C and 5% CO2. Cytotoxicity was measured to evaluate the influence of the dental wax on the quality of the seeded cells (Fig. 3). The determination of cytotoxicity was done according to the ISO 10993-5:2009 standard.
For optical evaluation the samples were stained with MTT (3-(4,5-dimethylthizol-2-y)-2,5-diphenyltetrazolium bromide). Viable cells can reduce MTT to formazan resulting in a color change from yellow to purple/blue which can be detected by absorption measurements with a photometer. Therefore, the culture medium was removed and then the cells were stained with 50 μl or 500 μl MTT solution (1mg/ml in DMEM) followed by incubation for 2 hours at 37° C and 5% CO2. Afterwards, the staining reagent was replaced by 50 μl or 500 μl isopropanol. The solution was transferred to 96 well plates and absorption was measured in the wavelength range of 560 to 650 nm. The optical evaluation of the cell morphology showed 100% normal fibroblasts for the test and control samples, as indicated in Figure 4.
For all measured samples, the cell viability was 85 ± 7 %.
Together with the evaluation of the morphology, the Coral Life workflow clearly shows that the SampLink chambers act as the optimal tool for keeping your cells of interest under the specific physiological conditions needed throughout any live-cell experiment.