Why use cell-culture inserts?
Permeable supports for cell culture, also called cell-culture inserts, are used to better replicate a 3D environment as would be experienced by cells in vivo [1,2]. Unlike the flat surfaces of dishes, flasks, or well plates, both the apical (top) and basolateral (bottom side) cellular surfaces are exposed to the culture environment within cell-culture inserts. These inserts are put into well plates and are commercially available with a variety of membrane types, coatings, customized surfaces, and pore sizes and densities. Cell-culture inserts are also used for studying cell transport, secretion, diffusion, migration, and tissue engineering.
Here the goal was to use cell-culture inserts to generate brain capillary endothelial-like cells using human induced pluripotent stem cells (iPSCs) [3,4]. These endothelial-like brain cells, along with astrocytes, pericytes, and neurons, constitute the blood–brain barrier (BBB). The generation of high-quality and accurate in vitro evaluation systems for the BBB is important for the screening of therapeutic agents against neurodegenerative and BBB-related diseases.
Conventional methods for checking cells in cell-culture inserts
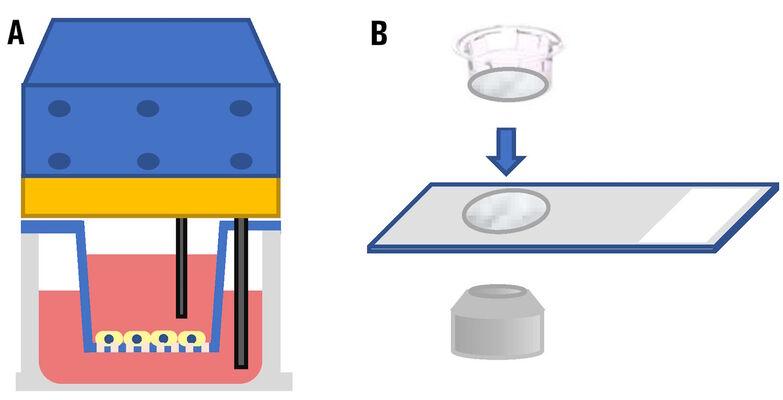
Until recently, if users needed to evaluate the cells in cell-culture inserts set in multi-well plates during an experiment, then there were essentially 2 ways to do that. Either they would use a dedicated electrode to check the resistance of the cultured cells or remove the membrane covering the insert, take out the insert, and then observe it on a glass slide using a microscope (refer to figure 1).
New rapid, reliable way to check cells in cell-culture inserts
Now, cells in inserts set in a multi-well plate can be checked efficiently via 3D microscopic imaging using a THUNDER Imager (refer to figure 2).
The THUNDER Imager takes advantage of high-performance objective lenses that can achieve high resolution and have long working distances. Leica objectives with a magnification of 16x, a numerical aperture (NA) of 0.6, and a working distance of 2.5 mm (refer to figure 3) can be used with the THUNDER Imager to observe live cells in the cell-culture inserts while still set in the multi-well plate.
Live-cell THUNDER imaging with inserts
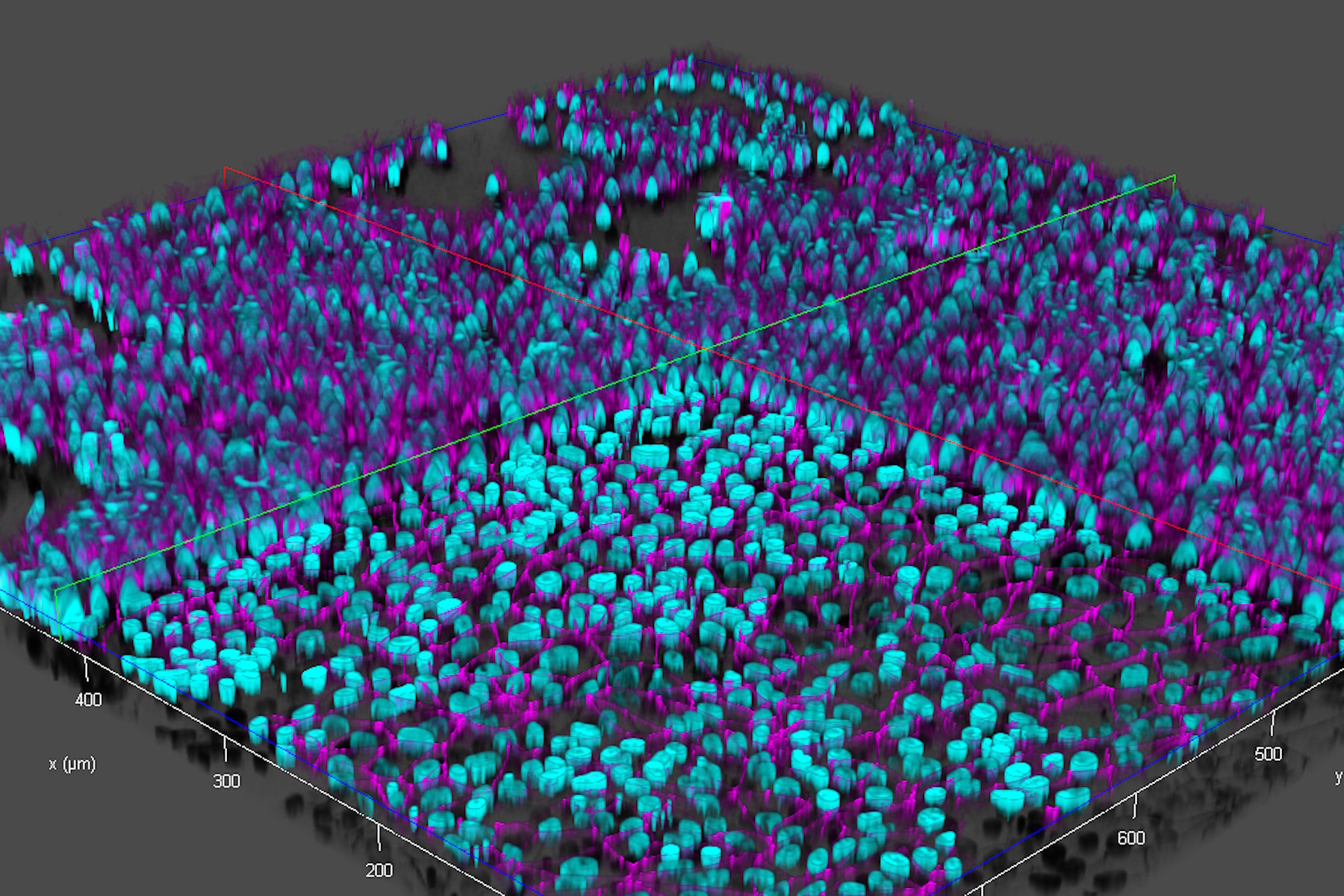
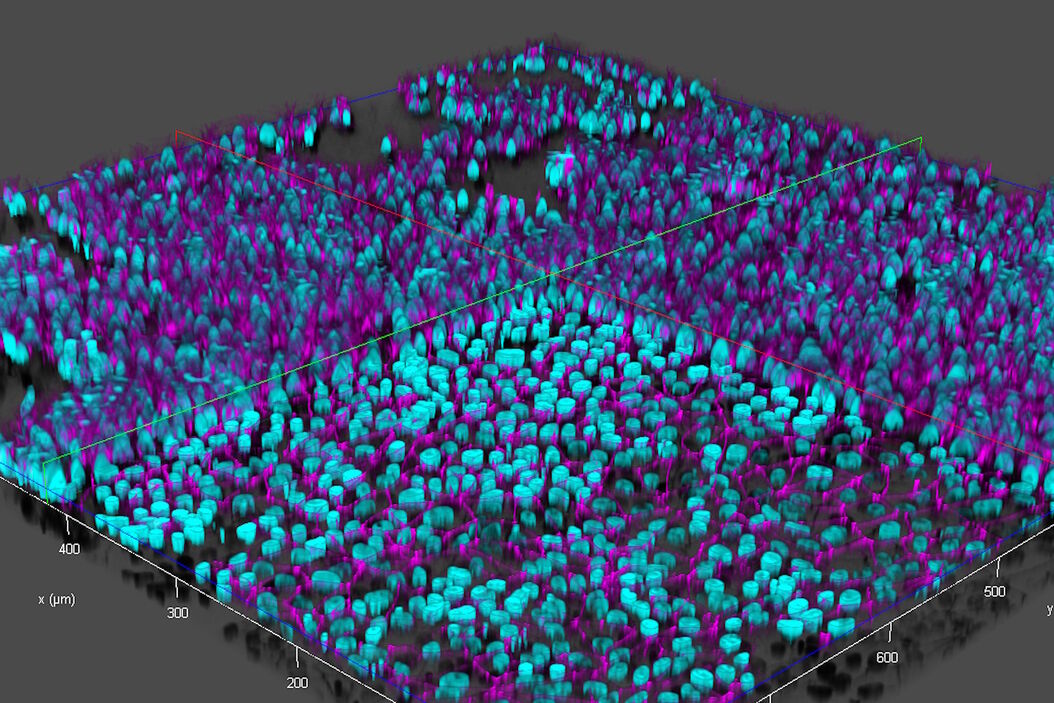
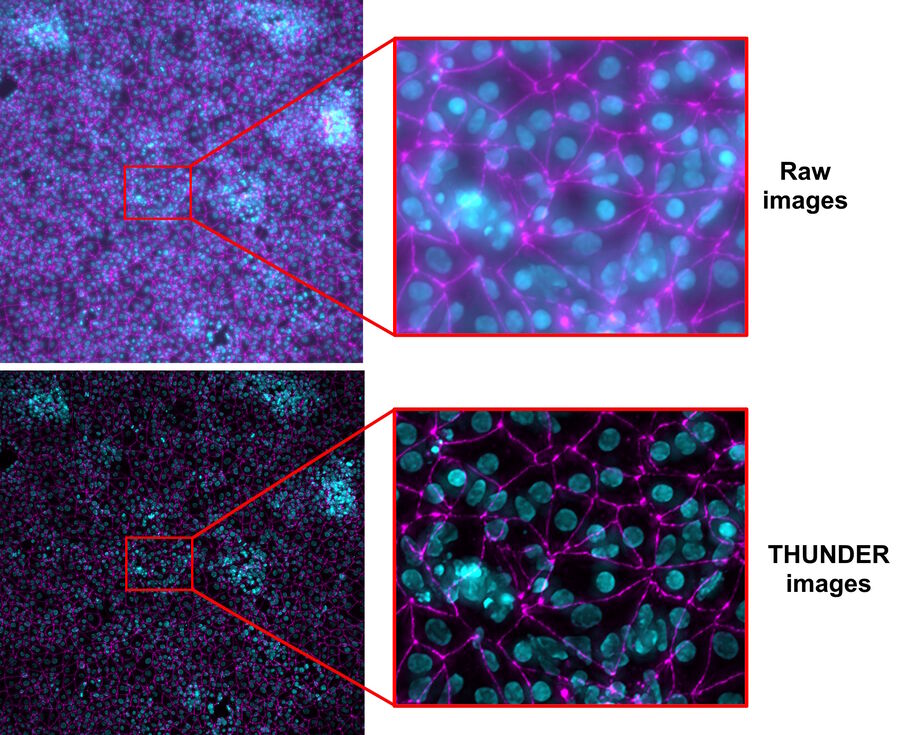
Brain-capillary endothelial-like cells derived from human iPSCs (induced pluripotent stem cells) were used for this study. The iPSC-derived endothelial-like cells, stained with DAPI to mark nuclei (cyan) and Alexa Fluor 488 to mark tight junctions (magenta) [5], were cultured in inserts which were set in a 12-well plate (inserts and well plate from Corning) [3,4]. For more details about cultivation and preparation of the cells, readers can refer to references 3 and 4.
Ultra-high-speed and high-resolution images of live endothelial-like cells in inserts and a multi-well plate were acquired with a THUNDER Imager:
- A fast Z-stack, where 70 focal planes over the vertical (Z) direction are acquired in about 20 seconds while alternating between 2 wavelengths with a high-speed filter (refer to figure 4);
- Scattered light present in the raw images of the live-cell samples were removed from the THUNDER images with Computational Clearing [6,7] (refer to figure 5).
In comparison to a conventional widefield microscope, when using the THUNDER Imager Live Cell or 3D Assay, the iPSCs grown in cell-culture inserts were checked about 3x faster, because there is no need to remove the inserts from the multi-well plate for microscope imaging.
Conclusions
The results show that rapid and reliable checking of live cells in cell-culture inserts set in a multi-well plate is possible with a THUNDER Imager . The cells imaged were brain-capillary endothelial-like cells derived from human iPSCs (induced pluripotent stem cells). The workflow was made easier and about 3x less time is needed to check the cells. This benefit results from the fact that inserts must not be retrieved from and then set back into the multi-well plate when checking the cells with THUNDER imaging .