Basics of fluorescence assays
Multiwell plates are very useful for experiments which demand a higher throughput. They enable users to collect data for 384, 96, or 24 different experiments (samples) which are performed in parallel [1]. Modern assay kits are typically based on fluorescent probes or dyes labeling proteins of interest. The fluorescently labelled sample in each well plate is then analyzed with fluorescence microscopy [2,3]. Fluorescence multiwell plate assays are used for a large variety of applications in the fields of neuroscience, cancer research, and developmental biology where a high throughput makes it more practical to run a lot of assays. Fortunately, kits for many of these fluorescence assays are commercially available which helps to greatly simplify the sample preparation, saving users a lot of time and effort.
Apoptosis and the Caspase proteins
Apoptosis, programmed cell death, occurs during organism embryo development to eliminate unwanted cells and during healing in adults to rid the body of damaged cells and help prevent cancer [4-6]. The caspases are a large family of cysteine proteases which play an essential role in the initiation and execution of apoptosis [5,6]. Initiator caspases help initiate apoptosis while the executioner caspases carry out mass proteolysis. Caspase-3 and caspase-7 are both executioner caspases [5,6,7]. During apoptosis, caspase-3 has a role in coordinating the destruction of cellular structures and caspase-7 in cell detachment [7].
Caspase assays
Caspase assays allow studies of the early phases of apoptosis. Here the dose-dependent activation of caspase-3 and capase-7 by cytotoxic drugs, like staurosporine, are investigated. To eliminate random effects, multiple assay samples under the same conditions, i.e., with different concentrations of one drug or combinations of multiple drugs, are examined. Statistically significant results typically require experiments to be repeated several times.
Challenges for caspase assays
The typical readout of a caspase assay is the information of when the caspase activation cascade is started. However, questions about other morphological or activation reactions of single cells remain unanswered. Adding more fluorescent stains to such an assay allows more information to be obtained concerning, for instance, the cytoskeleton behavior during apoptosis or the cytochrome-c mediated assembly of apoptosome leading to the activation of the caspase-3- and caspase-7-cleavage pathway [8]. It can be difficult to collect the signals of all fluorescent labels in the assay quick enough so that the allotted time interval to acquire data over the whole sample carrier is achieved. Secondly, separating the signals from up to four fluorescent labels in a rapid and reliable way can be challenging. Finally, correlation of the data with caspase activity is not straightforward when they are not acquired simultaneously.
Due to these challenges, there are often concerns when performing caspase assay experiments, particularly when combining additional markers for extended information gain. Context and morphological data may go missing or are not spatiotemporally correlatable, i.e. with respect to position and time. Also, crosstalk between fluorescent labels can lead to misinterpretation of results.
At present, a significant number of research labs may not have readily available all the instrumentation and microscopes needed for work involving these assays. This problem is usually resolved with access to shared facilities used by multiple research groups and users, but that can mean long waiting times before the needed instruments are available.
These challenges can lead to delays in acquiring the important, quantifiable results that lead to fundamental breakthroughs and the gaining of new insights.
Introducing Mica
Mica, the world's first imaging Microhub, unifies everything a researcher needs in one completely controlled, highly flexible environment, supercharging microscopy workflows to get meaningful scientific results faster. By using this Microhub, you benefit from:
- Access for all: Mica allows the setup of complex assay readouts even for inexperienced users
- No constraints: Mica allows users to image 4 labels simultaneously, with 100% spatiotemporal correlation
- Radically simplified workflows: Mica combines the collection of 4 markers and AI-powered data analysis, all with significantly fewer workflow steps
Methods
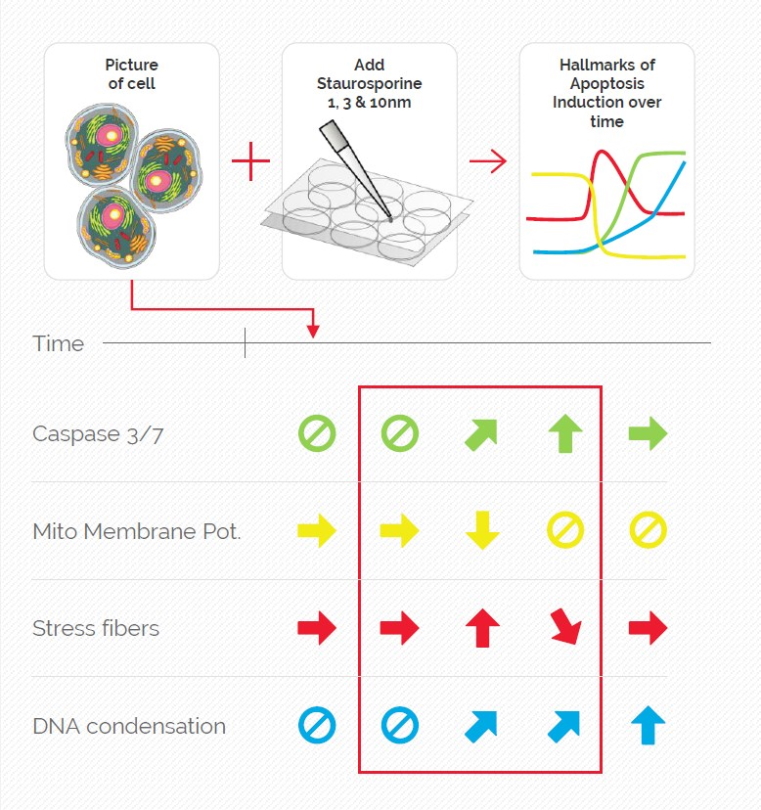
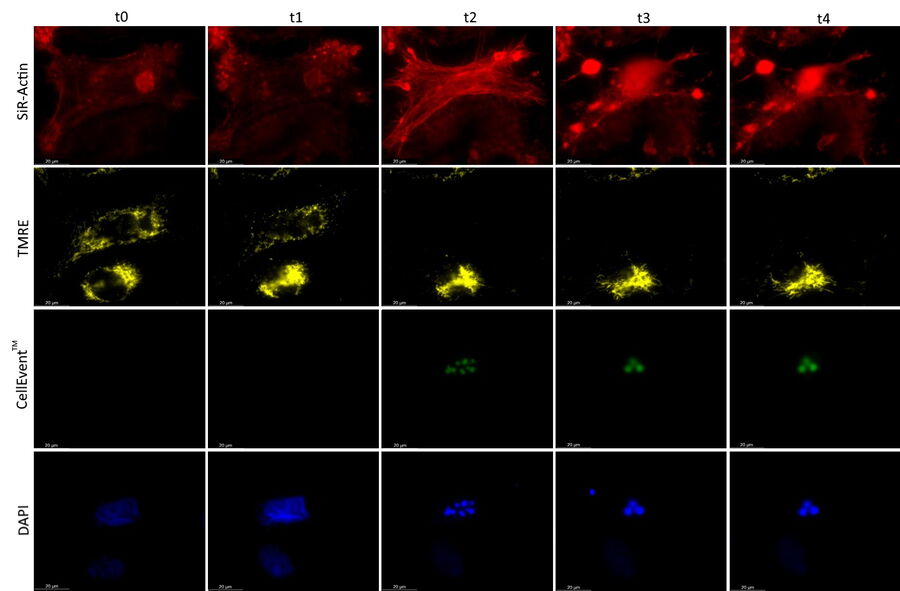
Mica is used for this key application with fluorescence multiwell plate assays concerning the role of caspase-3 and caspase-7 in the activation of apoptosis (figure 1). Samples were prepared from the U2OS cell line and finally treated with staurosporine to induce apoptosis. This experiment combined multiple readouts which all relate to the early phases of apoptosis.
Results
The results shown in figure 1 show a staurosporine induced activation of caspase-3 and caspase-7 which is preceded by stress-fiber formation and the depletion of the mitochondrial membrane potential.
The absolute spatiotemporal correlation of 4 fluorescent markers, which enable the monitoring of early apoptosis induction, was achieved with Mica (refer to video 1 and figure 2). The formation of stress fibers coinciding with the loss of mitochondrial membrane potential at the beginning of caspase-3 and caspase-7 activation was observed. DNA condensation directly followed the caspase activation.
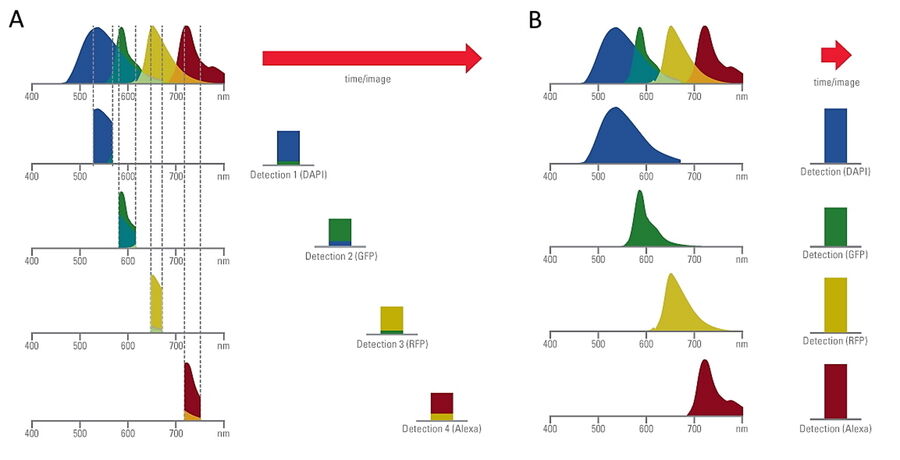
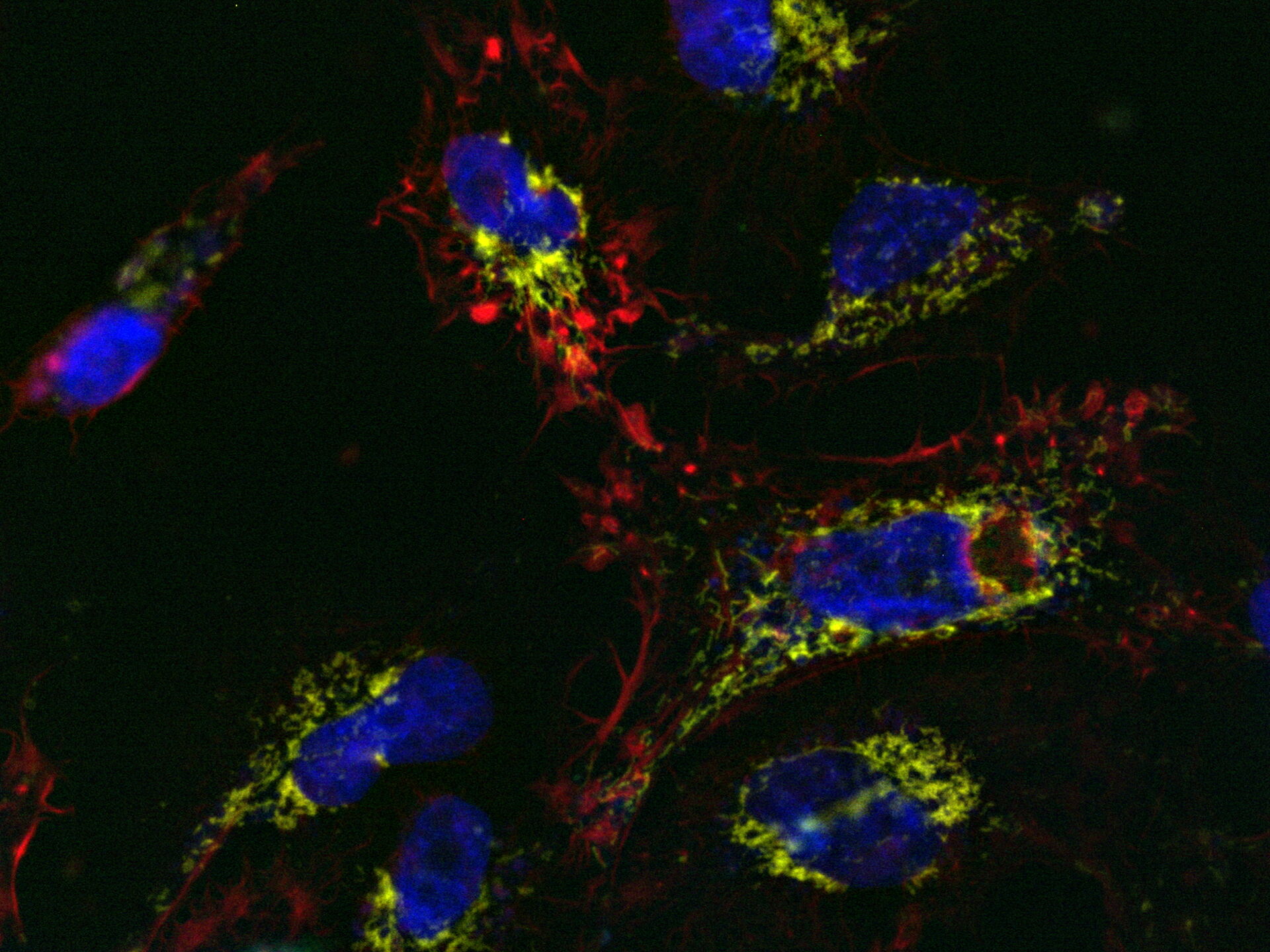
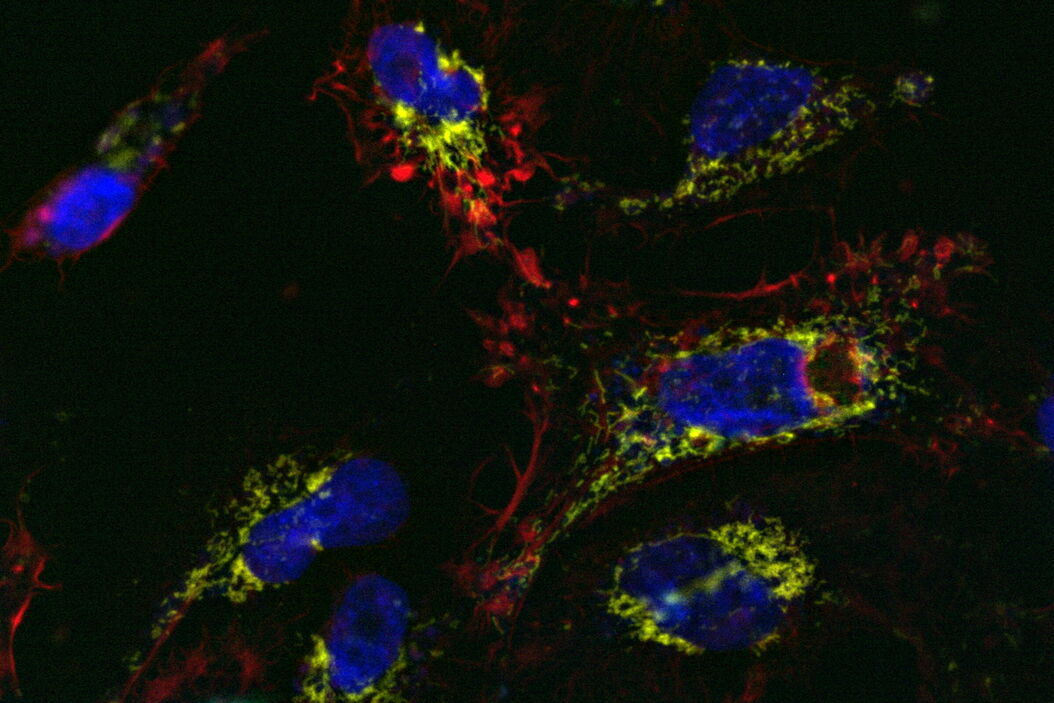
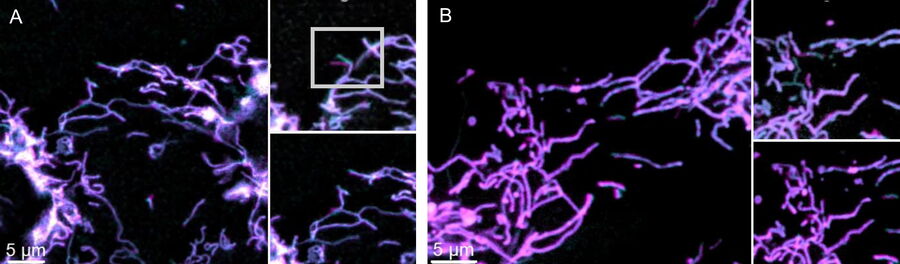
Comparison of spatiotemporal multicolor acquisition between conventional widefield microscopy and Mica is shown in figure 3. When studying mitochondrial dynamics, simultaneous fluorescent label detection eliminates spatiotemporal artifacts when visualizing mitochondrial structure and membrane potential. When using traditional widefield imaging, spatiotemporal artifacts resulting from sequential acquisition can falsify measurements and prevent the correlation of structure and function. Mica offers FluoSync which enables simultaneous acquisition and ensures spatiotemporal correlation. No artifacts are acquired and the membrane potential is correctly correlated with the mitochondrial structure.