What is Parkinson’s disease?
Parkinson’s disease (PD) is a neurodegenerative disorder which decreases the quality of life of affected individuals. Famous examples of people who have or had Parkinson’s are the athlete Muhammed Ali and the actor Michael J. Fox. Causes of the disease are manifold, e.g., genetic (case of Michael J. Fox) or due to physical influence (case of Muhammed Ali). Treatments until now mainly focus on symptom minimization which is achieved by L-DOPA (a dopamine predecessor) medication.
The underlaying cause of the disease is selective dying of a population of dopaminergic (DA) neurons in the midbrain or substantia nigra (SN, black substance) to be precise [1,2]. First phenotypes are visible after a loss of more than 60% of dopaminergic neurons. Thus, a comparison of midbrain tissue from postmortem Parkinson’s patients based on age and gender matched controls is like comparing apples with bananas. Therefore, surviving dopaminergic neurons from the substantia nigra pars compacta have to be analyzed one by one to find differences between these specific dopamine producing cells.
There are several theories on the origin of the disease. For that reason, Parkinson’s disease can be compared to a puzzle. Many pieces have already been found and some of them have been put together, but no one still knows what the whole picture looks like. The significance of some of the pieces has not been worked out yet, neither does anyone know how many pieces of the puzzle still have to be found before a full picture of Parkinson’s can be presented to have a clear understanding of the disease.
Dopaminergic neuron isolation in spatial context and Parkinson’s research
Typical workflows and experimental procedures used
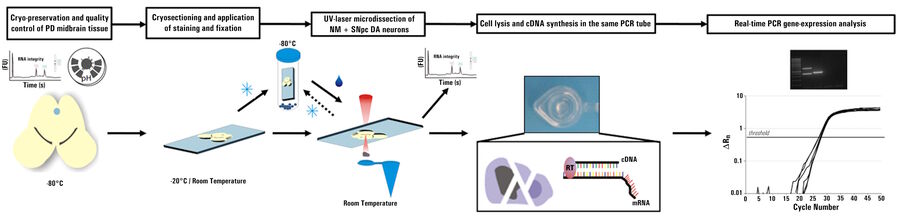
Animal models used to study Parkinson’s disease are rather rare, as this disease usually appears at a later age and, as a result, murine (mice and rats) models have no clear phenotype comparable to humans. Studies in monkeys, where Parkinson’s can be introduced by drugs, even in younger animals, are expensive and require special conditions which are not accessible for many researchers. Besides those challenges, the activity of single neurons is often investigated with electrophysiology. This approach requires vital brain sections which cannot be acquired from humans. Thus, a different way to selectively analyze dopaminergic (DA) neurons is needed. For this reason, the method of laser microdissection (LMD) comes into play [3].
Analysis and understanding of the data and results
By correlating the molecular biology results from individual neurons, where repatching was performed with a needle to extract the content for downstream analysis, as well as from LMD-dissected neurons, it can be proven that the results of both collection methods are comparable. Also, LMD allows researchers to collect individual neurons from postmortem human midbrains and, more specifically, from the SN where the DA neurons can be easily identified due to their neuromelanin content which makes them appear black. Using LMD, an expression pattern of individual surviving DA neurons from postmortem Parkinson’s patients and matching controls can be analyzed with the goal of finding the molecular level difference of the two groups. This type of investigation can help understand the cause or consequences of Parkinson’s.
Challenges when studying dopaminergic neurons in the substantia nigra
Analysis of different postmortem specimens are like “snapshots” taken at a specific stage of Parkinson’s disease. These “snapshots” can be compared with each other, but these specimens cannot be used to detect whether a changed gene expression is the result or cause of the disease. Further research is needed on this subject. Also, considering the fact that gene expression in Parkinson’s patients is only altered in dopamine-releasing neurons, then for research on the Parkinson’s disease puzzle, only these relevant cell types should be analyzed. If, by accident, other cell types are added to the analyte, they would decrease the quality of the results.
Laser microdissection
Laser microdissection (LMD), also known as laser capture microdissection (LCM), is a contact- and contamination-free method for isolating specific single cells or entire areas of tissue within spatial context from a wide variety of tissue specimens. The thickness, texture, and preparation of the original tissue are relatively unimportant.
The dissectate is then available for further molecular biological methods, such as next generation sequencing (NGS), which includes RNA sequencing, quantitative polymerase chain reaction (qPCR), real-time qPCR, proteomics, metabolomics, lipidomics, and multi-omics techniques. LMD is now used in a large number of research fields, e.g., pathology, neurology, cancer, plant analysis, forensics, and climate. Additionally, the LMD is used for manipulation of cell cultures or micro-engraving of coverslips.
LMD is useful for the optimization of workflows for genomics (DNA), transcriptomics (RNA), and proteomics (proteins), as it allows pure starting material to be precisely extracted and then collected from tissues for analysis under visual control.
Results with LMD
The analysis of single cells frequently shows different results compared to those obtained from a complete tissue examination [3-6]. Studies have shown that the expression of certain microRNAs is changed in the tissue of Parkinson’s patients.
When microdissected cells are examined in parallel, it is found that the microRNA expression is not changed at the cellular level. This tissue artifact was detected with the aid of laser microdissection.
The use of a LMD system enables contact-free dissection of single cells or, if necessary, larger areas of tissue. The dissected material is captured in the cap of a tube and can be processed immediately. Refer to figures 2, 3, and 4 below.
Future challenges for Parkinson’s research
At present, there are no tests for diagnosing Parkinson’s disease at an early stage and there are many variations of the disease. Also, there are diseases with similar symptoms. Therefore, a certain percentage of Parkinson’s cases are diagnosed wrongly or not diagnosed at all.
The prerequisite for successful treatment of Parkinson’s would be effective early diagnosis. If the disease could be diagnosed at an initial stage in which the neurons are just beginning to degenerate, it might be possible to prevent progressive neuron degeneration and stop the disease altogether.
The identification of biomarkers in blood or cerebral fluid is currently a major research focus. There are genes that are not only expressed in the brain, but ubiquitously in all cells. If the expression of these genes were changed in the dopaminergic neurons of a Parkinson’s case, it would be possible to examine more easily accessible tissue for diagnostic purposes.
Perhaps in the near future a combination of laser microdissection (LMD) with AI (artificial intelligence) and even mass spectroscopy could lead to even further breakthroughs for a better understanding of Parkinson’s disease.
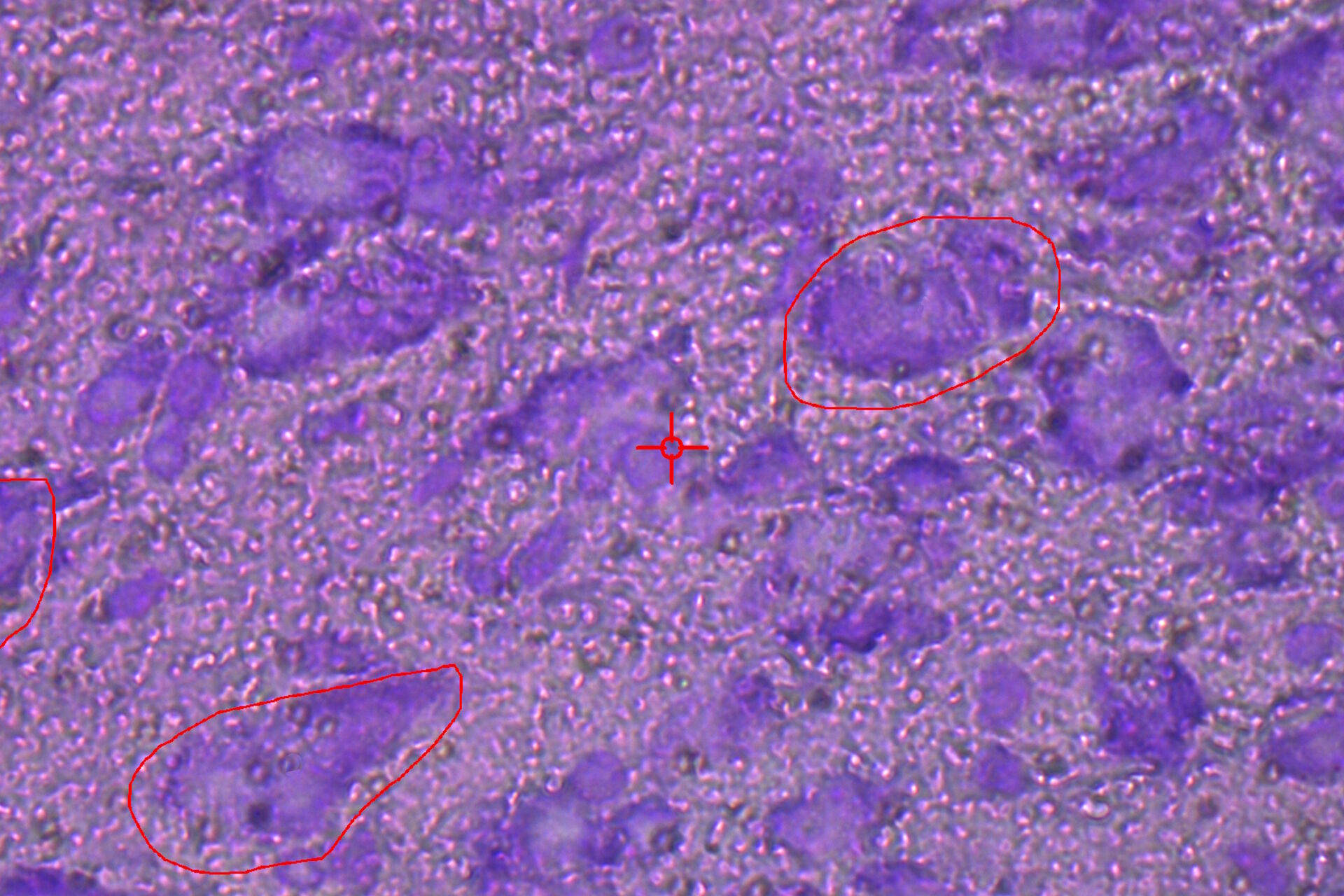
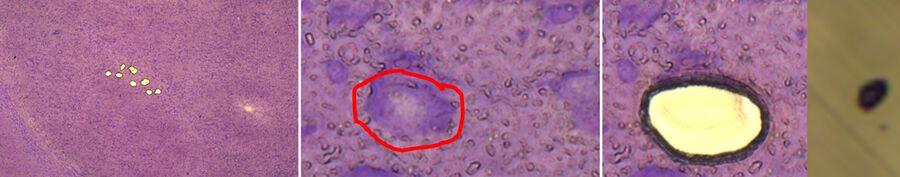
![LMD and mRNA-expression analysis of individual SN DA neurons from human PD and control postmortem brains Fig. 3: LMD and mRNA-expression analysis of individual SN DA neurons from human PD and control postmortem brains. Pools of neuromelanin-positive [NM(+)] neurons were isolated via LMD from cresyl-violet-stained horizontal midbrain cryo-sections from PD (A) and control brains (B).](/fileadmin/_processed_/c/a/csm_LMD_and_mRNA-expression_analysis_of_SN_DA_neurons_f4fe1527ea.jpg)
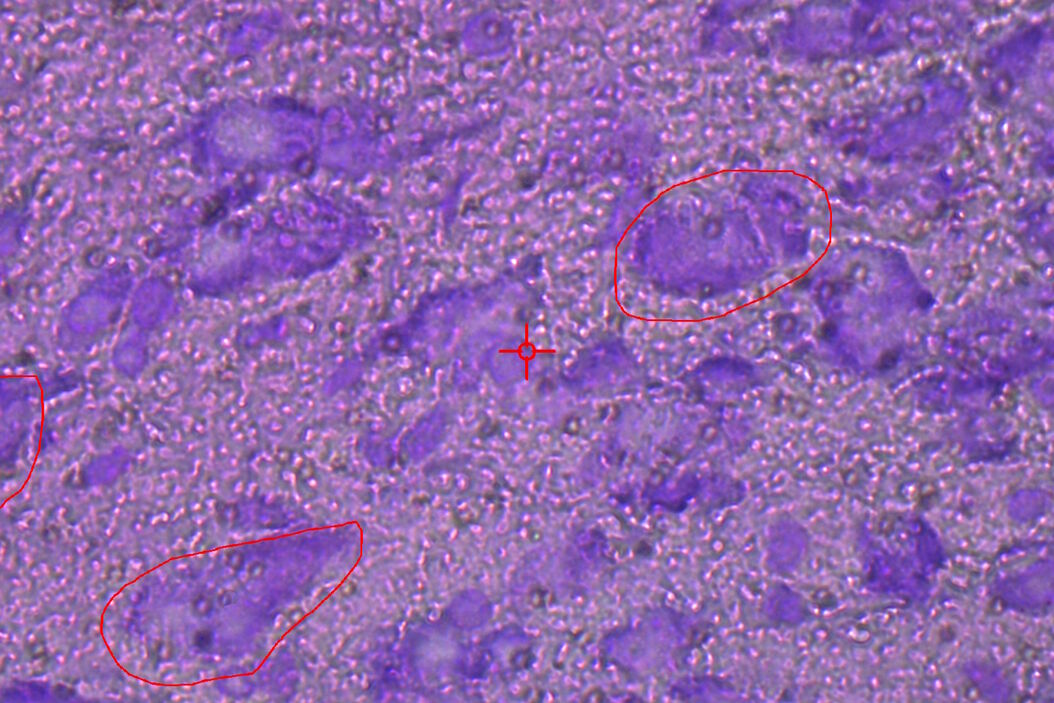
![Cresyl-violet-stained mouse brain cryo-section before (A) and after (B) LMD. Fig. 4: Cresyl-violet-stained mouse brain cryo-section before (A) and after (B) LMD [7].](/fileadmin/_processed_/d/c/csm_Cresyl-violet-stained_mouse_brain_cryo-section_before_and_after_LMD_92a8092eda.jpg)