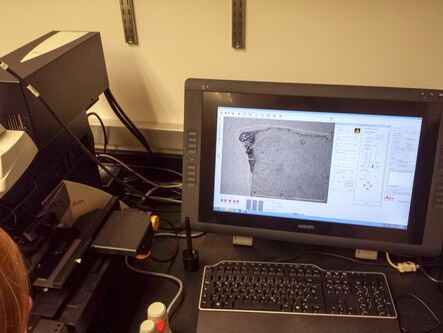
Material – lab inventory
Leica LMD system
- Cryostat (e.g. Leica CM1850 UV)
- Pipet 10–1,000 µl
- Pipet 2–20 µl
- Centrifuge for tubes
- Tweezer
- Tweezer and brush for cryostat
Material – chemicals and consumables
Qiagen RNeasy Micro kit
- A bag of 50 ml Falcon tubes
- Pure ethanol (EtOH), molecular biology grade
- Molecular biology grade water
- Cresyl violet
- Aluminum foil
- 0.5 or 0.2 ml thin walled PCR tubes with flat cap suitable for the LMD stage collection holder
- Pipet tips (for 1 ml pipet and 20 µl pipet)
- Syringe with sterile filter tip
- Ice
- Blade for cryotome
- Parafilm
- Leica LMD slides (e.g. 4 µm PEN frames)
- Silica bags
- Kimwipes
Preparation upstream
1 % cresyl violet (CV) solution in 100 % ethanol (EtOH): add 500 mg cresyl violet powder into a 50 ml Falcon tube and add 100 % EtOH to fill 50 ml. Should be prepared 1 week prior to usage. Put Falcon in fridge, cover with aluminium foil (cresyl violet is light-sensitive) and shake gently every day.
EtOH row for fixation and wash steps:
- 2 times 75 % EtOH: fill 37.5 ml EtOH into 50 ml Falcon tube and add molecular biology grade water to 50 ml
- Place one 75% EtOH Falcon into –20 °C freezer
- 95 % EtOH 47.5 ml EtOH into 50 ml Falcon tube and add molecular biology grade water to 50 ml
- 100 % EtOH 50 ml EtOH into 50 ml Falcon tube
- Ethanol row in Falcon tubes can be (re-)used for up to 3 days.
Important note:
- Seal Falcons with Parafilm for storage → this will prevent EtOH from evaporating!
- Place LMD slides for 10 min under UV-C light → acivate the membrane for better section adhesion and sterilize the surface!
- Place 0.2 or 0.5 ml tubes with lid open under UV-C light for at least 30 min → sterilize and eliminate any RNA & RNase!
- Prepare three 50 ml Falcon tubes with some silica bags in the conus → The silica will keep the slides and section dry, working with RNA means humidity and moisture are your enemy, because they could activate RNases!
Cryosectioning
Place block with cryosample into Leica cryostat directly from –80 °C and let it equilibrate for at least 30 min at –19 °C.

After equilibration prepare sections of a suitable thickness (thickness should be chosen according to the diameter of cells of interest, for this test 10 µm sections were prepared).
Place one (curled) section directly from cryo into a 0.5 ml tube and immediately add 350 µl RLT buffer (content of Qiagen Micro RNeasy kit). This will serve as positive control for quality and quantity.
Put the slide with the section into the Falcon with 75 % EtOH solution at –20 °C for 2 min (brief fixation). After 2 min briefly air-dry the slide and store it in a 50 ml Falcon with some silica bags in the conus (to keep it dry).
Repeat this mounting with another section and slide, but instead of storage add some drops of 1 % cresyl violet solution with the syringe through the sterile filter tip. Leave the staining solution on the section for 1 min. Afterwards, wash off the cresyl violet by dipping the slide briefly into 75 % EtOH, then 95 % EtOH and finally 100 % EtOH. Briefly air-dry the slide and store it in a 50 ml Falcon with some silica bags in the conus (to keep it dry).
Repeat the whole process with another section and slide.
Important note:
- Store slides in Falcon tubes with some silica bags in the conus and seal those with Parafilm → the silica will keep the slides and section dry, sealing protects against environmental moisture!
Store all tubes and Falcons with slides on ice. Let the slides equilibrate for 15 min at room temperature next to the LMD system in the sealed Falcon prior to usage.
LMD test slides
You should now have:
- 1 section in a tube with 350 µl RLT buffer (positive control)
- 1 section mounted on a slide and fixed with EtOH stored in a 50 ml Falcon with silica bags
- 2 slides with mounted sections fixed with EtOH and stained with CV, stored in a 50 ml Falcon with silica bags
The slide with the unstained section is used as another control. Therefore directly digest it from the membrane with RLT buffer and transfer it into a 0.5 ml tube (total amount of RLT buffer must be 350 µl, do not use all of it for digestion from the slide as it will rinse off the slide!).
One of the fixed and stained slides is used as another control. Therefore directly digest the section from the membrane with RLT buffer and transfer it into a 0.5 ml tube (total amount of RLT buffer must be 350 µl, do not use all of it for digestion from the slide as it will rinse off the slide!).
Leica LMD system for RNA analysis
The remaining fixed and stained slide is applied for LMD. Therefore, load the slide in the sample holder and load a 0.5 ml tube in the collection holder. Select the position of the loaded collector and mark the whole section for dissection with the LMD software, using the desired magnification. Start the laser and check after dissection that all the sections have been collected in the collection tube. If necessary, recut the section using Move+Cut to ensure all the sections are collected.
Unload the collection tube, carefully close the lid and briefly spin down the dissectates. Open the lid and add 350 µl RLT buffer (up to 65 µl RLT buffer can be added to the collection cap before cutting).
Load another collection cap and dissect empty membrane close to the area where the section was collected (roughly the same area size in µm²). Unload the collection tube, carefully close the lid and briefly spin down the dissectates. Open the lid and add 350 µl RLT buffer (up to 65 µl RLT buffer can be added to the collection cap before cutting). This tube will serve as a LMD negative control later on.
Important note:
- Up to 65 µl RLT buffer can be directly applied to the collection tube cap to protect the content of the dissectate from degradation directly after capture
Preparation and processing downstream from laser microdissection
- Add 44 ml to the bottle with RPE and check the checkbox to ensure that EtOH is added.
- Prepare a solution of 70 % EtOH: add 35 ml 100 % EtOH to a 50 ml Falcon and add 15 ml molecular biology grade water.
- Prepare a solution of 80 % EtOH: add 40 ml 100 % EtOH to a 50 ml Falcon and add 10 ml molecular biology grade water.
Extract RNA using the Qiagen Micro kit with the following slightly modified protocol:
- Have all tubes ready.
- Add 350 µl 70% EtOH to each tube (if necessary, transfer into another tube of suitable volume before adding 350 µl 70 % EtOH), carefully mix with pipet and directly transfer to red spin column.
Important note:
350 µl should be split up into 100 and 250 µl so as not to exceed the volume of the 0.5 ml tube. Mixing can be done directly at the column.
- Spin 15 s at 10,000 rpm.
- Discard the flow-through and add 350 µl RW1 to wash column
- Spin 15 s at 10,000 rpm.
- Discard the flow-through, place the column in a fresh 2 ml tube and add 500 µl RPE (EtOH added before) to wash column
- Spin 15 s at 10,000 rpm.
- Discard the flow-through and add 500 µl 80 % EtOH to wash column
- Spin 2 min at 13,000 rpm
- Discard the flow-through and place the column to a fresh 2 ml tube
- Spin 5 min with max. speed and lids open to dry the silica membrane of the column
- Elute in 14 µl water: carefully pipet 14 µl RNAse-free water into the middle of the column membrane and place the column in a fresh and labeled 1.5 ml tube
- Spin 1 min at 13,000 rpm
- Carefully pipet the flow-through back into the middle of the column membrane
- Spin 2 min at 13,000 rpm
The eluate should be stored at –80 °C or if possible immediately used for evaluation of quality and quantity.
Brief protocol summary
Samples:
A – sample picked directly from cryo (overall positive control)
B – sample fixed, picked by pipet (positive control, effect of EtOH fixation)
C – sample fixed and stained, picked by pipet (positive control, effect of EtOH fixation and staining)
D – sample fixed and stained, collected by LMD (effect of LMD)
E – empty membrane collected beside sample (negative control of LMD process/contaminations)
F – RLT buffer negative control of downstream purification
RNA extracted with Qiagen Micro kit:
- Add 350 µl RLT buffer
- Add 350 µl 70 % EtOH, mix with pipet and directly transfer to column
- Spin 15 s, 10,000 rpm
- Discard flow-through and wash with 350 µl RW1
- Spin 15 s, 10,000 rpm
- Discard flow-through and wash with 500 µl RPE (EtOH added before)
- Spin 15 s, 10,000 rpm
- Discard flow-through and wash with 500 µl 80% EtOH
- Spin 2 min, 10,000 rpm
- Discard flow-through
- Spin 2 min, max. speed with lids open to dry membrane
- Elute in 14 µl water*
- Spin 1 min, full speed
- Use eluate and put it back in the column
- Spin 1 min, full speed
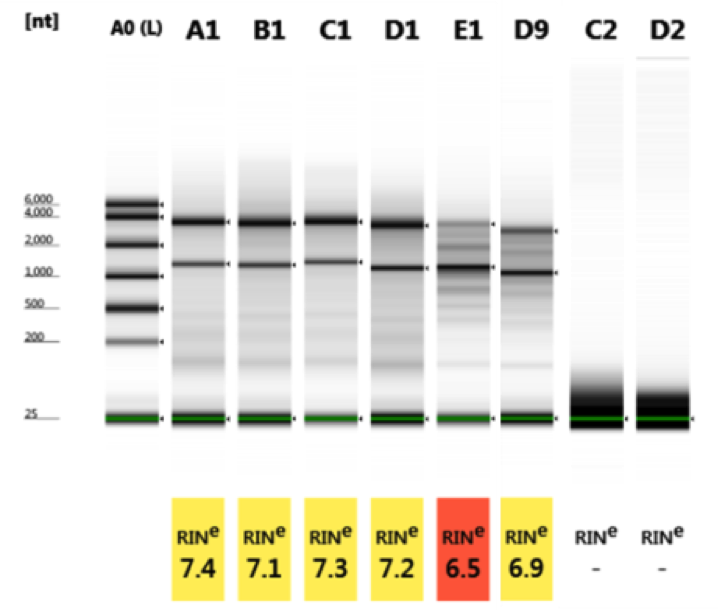
| Well | RIN® | 28S/18S (height) | 28S/18S (area) | Conc. (ng/µl) | Sample description | Alert | Observations | Total RNA area | rRNA area |
|---|---|---|---|---|---|---|---|---|---|
| A0 | – | – | – | 137 | Ladder | – | – | ||
| A1 | 7.4 | 1.4 | 1.9 | 69.1 | A | 2.79 | 0.61 | ||
| B1 | 7.1 | 1.3 | 1.9 | 84.5 | B | 3.41 | 0.76 | ||
| C1 | 7.3 | 1.4 | 2.0 | 127 | C | 5.13 | 1.26 | ||
| D1 | 7.2 | 1.0 | 2.0 | 83.6 | D | 3.38 | 0.78 | ||
| E1 | 6.5 | 0.4 | 0.4 | 110 | E | 4.44 | 0.98 | ||
| D9 | 6.9 | 0.7 | 0.9 | 67.3 | D | 2.68 | 0.59 | ||
| C2 | – | – | – | 7.36 | K | ! | RNA concentration outside recommended range for RINe | 0.30 | – |
| D2 | – | – | – | 6.57 | L | ! | RNA concentration outside recommended range for RINe | 0.27 | – |
| Sample name | Material | Date | Time | A260 concentration (ng/ul) | A260 (10 mm) | A280 (10 mm) | A260/A280 | A260/A230 |
|---|---|---|---|---|---|---|---|---|
| blank_6 | RNA | 11.12.2014 | ###### | 0 | 0 | 0 | – | – |
| A | RNA | 11.12.2014 | ###### | 32.42 | 0.81 | 0.41 | 1.98 | 0.13 |
| B | RNA | 11.12.2014 | ###### | 48.18 | 1.2 | 0.58 | 2.07 | 0.1 |
| C | RNA | 11.12.2014 | ###### | 71.01 | 1.78 | 0.89 | 2 | 1.51 |
| D | RNA | 11.12.2014 | ###### | 48.32 | 1.21 | 0.54 | 2.24 | 0.03 |
| E | RNA | 11.12.2014 | ###### | 64.65 | 1.62 | 0.73 | 2.2 | 0.06 |
| D | RNA | 10.12.2014 | ###### | 41.79 | 1.04 | 0.47 | 2.23 | 0.03 |
| K | RNA | 11.12.2014 | ###### | 1.72 | 0.04 | 0.02 | 1.97 | 0.02 |
| L | RNA | 11.12.2014 | ###### | 0.74 | 0.02 | 0.01 | 2.91 | 0.02 |
Quality
All samples (A–D) with different treatments have almost the same quality (RIN 7.1–7.4). No influence on EtOH fixation, staining and Leica LMD dissection detectable.
Cryo-storage of slide in a Parafilm-sealed 50 ml Falcon tube at–80 °C overnight, gently thawed step-wise for 20 min in –20 °C freezer, 20 min in 4 °C fridge and 15 min at room temperature prior to re-opening the Falcon tube led to comparable results after Leica LMD dissection as well (sample E1&D9, RIN 6.9 and 6.5).
All negative controls were empty.
Quantity
All samples (A–D) with different treatments have almost the same quantity, only sample C (fixed, stained and picked directly from slide) shows higher quantity than positive control (sample A). No influence from EtOH fixation, staining or Leica LMD dissection detectable.
Cryo-storage of slide in a Parafilm-sealed 50 ml Falcon tube at –80 °C overnight, gently thawed step-wise for 20 min in –20 °C freezer, 20 min in 4 °C fridge and 15 min at room temperature prior to re-opening the Falcon tube led to comparable results after Leica LMD dissection as well (sample E1 and D9, slightly higher quantity after storage).
All negative controls were empty.
Different measurement methods led to different results → common inaccuracy of measurements.
To summarize, these data clearly indicate that the workflow does not affect the RNA quality or quantity.
Acknowledgement
I would like to thank Dr. Randolph-Habecker for hosting the LMD workshop.