Introduction
Intraoperative optical coherence tomography (intraoperative OCT) is a microscope-integrated system designed to assist ophthalmic surgeons by providing helpful information for diagnosis and surgical procedures. This valuable technology can be used in treating a variety of ophthalmic diseases and has been increasingly used in the operating room (OR) [1-3].
Retina surgery has tremendously evolved with emergence of digital 3D heads-up viewing systems. These technologies have led to improvement in image quality, depth of focus, field of view, and also important ergonomic benefits for retina surgeons compared to the standard operating approach of viewing the procedure through the binoculars of a surgical microscope [4-6].
The EnFocus intraoperative OCT system is built into the Proveo 8 ophthalmic microscope (Leica Microsystems). It is a high-resolution device which can be used during both anterior and posterior segment surgeries. The Leica OCT solution can be used with retina wide-angle viewing systems such as the RUV800 (Leica Microsystems) and BIOM (Möller-Wedel, Germany).
Real-time acquired OCT scans can be viewed in video mode or frame by frame via the monitor that is part of the EnFocus OCT cart solution. Also, still images from scans can be made.
In addition, the surgeon can also use this system to visualize the OCT image directly on the microscope's monitor, as well as on external monitors in the OR, thanks to the latest EnFocus OCT solution from Leica fully built into the Proveo 8.
The surgeries described in this document were performed with the 3D digitally assisted system (NGENUITY® Alcon). The images were displayed on a single screen using a HDMI standard cable connection. Thus, the surgeon was able to analyze the OCT images using a picture-in-picture view, which provided larger images as the screen could be split in two. When used together, intraoperative OCT and heads-up 3D visualization systems can improve the surgeon’s decision-making regarding optimal surgical strategies aiming to improve surgical outcomes and, consequently also improving outcomes for patients.
In recent years, intraoperative OCT has become an integral part of the surgical workflow in eye surgery and herein, we share our experience of using this technology in our daily cases. To give an overview of the most interesting cases that we have performed with the EnFocus intraoperative OCT built into the Proveo 8 microscope (Leica Microsystems) we have selected cases of macular hole (MH) repair, subretinal fibrosis and submacular hemorrhage treatment and autologous retinal pigment epithelium (RPE) transplantation.
Case collection
1. Macular hole repair in highly myopic patients
The surgical success rate of repair of idiopathic macular holes (MH) smaller than 250 µm is about 98%, while for larger holes (400 µm or more), the closure rate is over 90%.21 In contrast, when highly myopic eyes are involved, MH with retinal detachment has a low surgery closure rate compared to idiopathic MH. Studies have shown considerable variations in closure rates, ranging from 60-100%, while some patients require more than one procedure to achieve closure of the MH [6-10,14,15].
For patients with retinal detachment and MH, the decision concerning how to treat the remaining subretinal fluid is not always clear. Specifically, there may be some uncertainty around removing the remaining subretinal fluid, and whether it should be aspirated or left. If aspiration of subretinal fluid is chosen, there may also be uncertainty around the way it should be performed – through the macular hole or a retinotomy. In addition, the aspiration procedure also has a certain risk. As the surgeon moves the cannula too close to the fluid, there is a risk of iatrogenic damage to the edges of the macular hole, or even to the RPE. With the real-time intraoperative OCT visualization, surgeons can minimize the risk of damaging these structures during aspiration of the subretinal fluid.
The necessity and the duration of postoperative face-down positioning are still under debate.11 Lorusso et al. affirmed that intraoperative confirmation of macular hole closure with intraoperative OCT could serve as a potentially useful tool for prescribing less than 24h of face-down positioning [12].
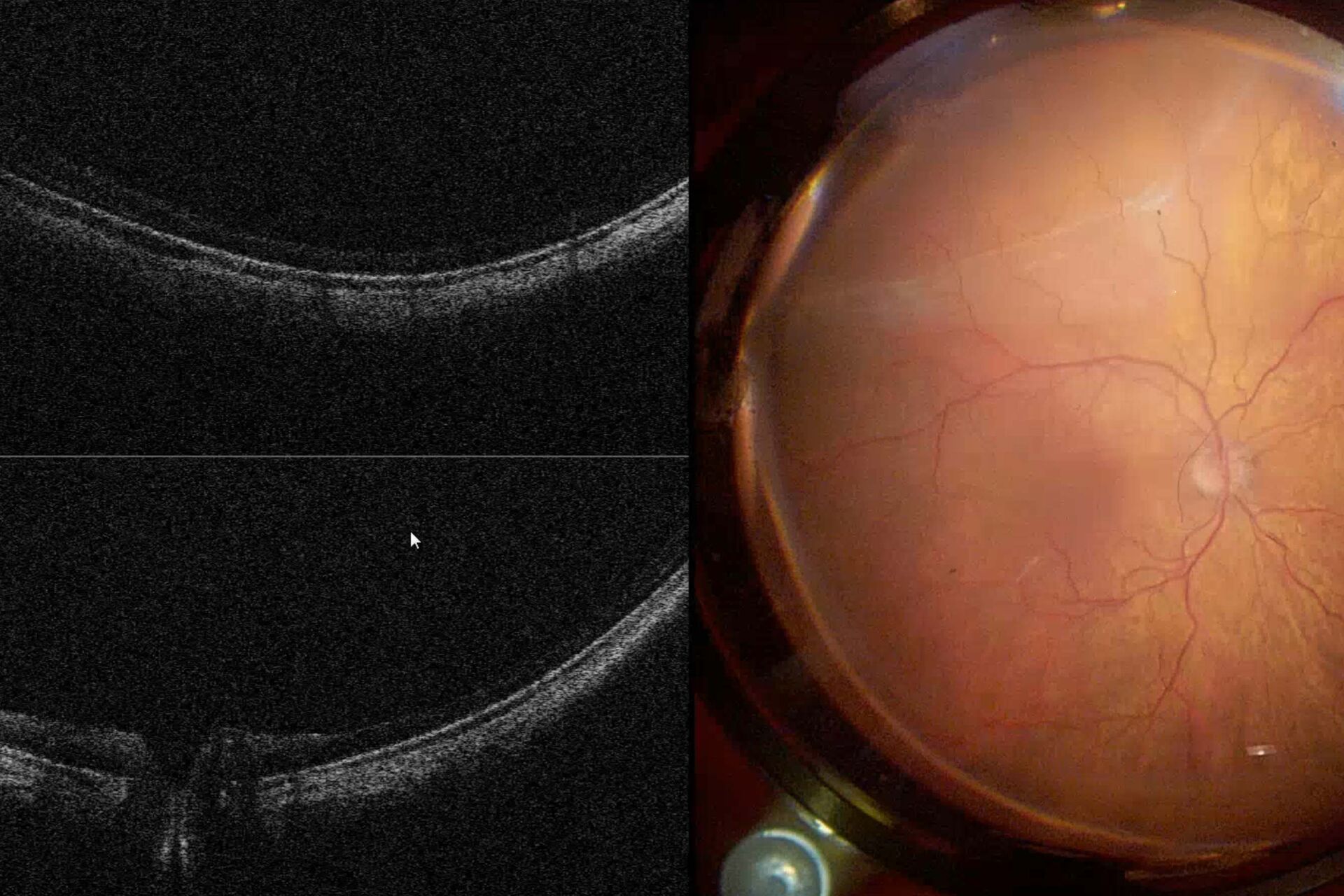
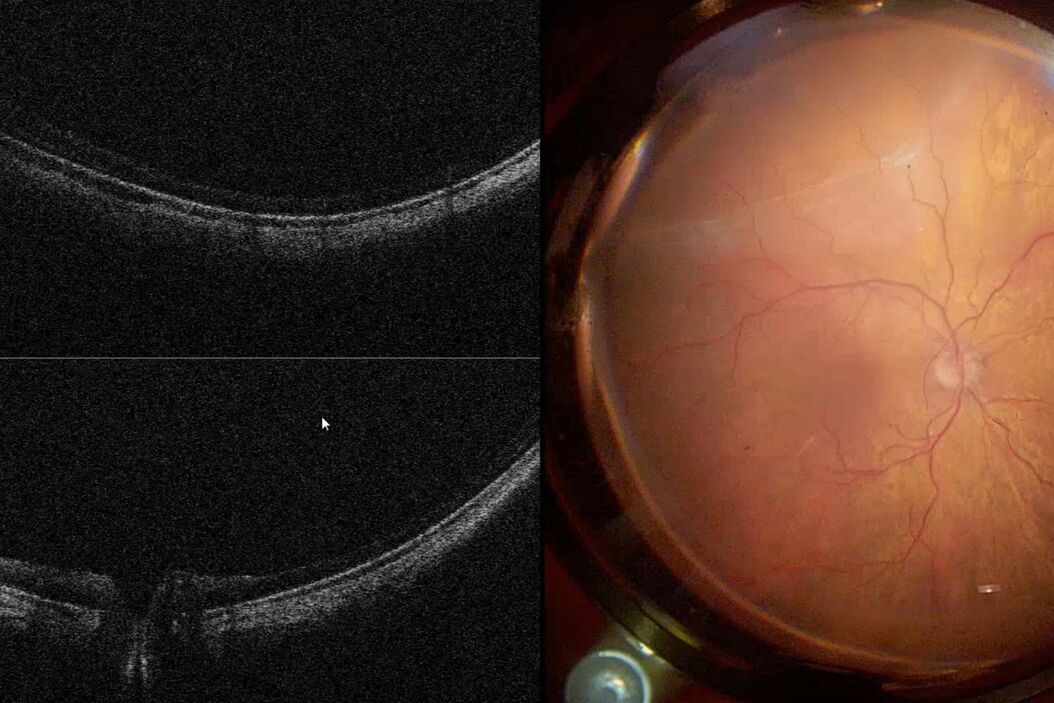
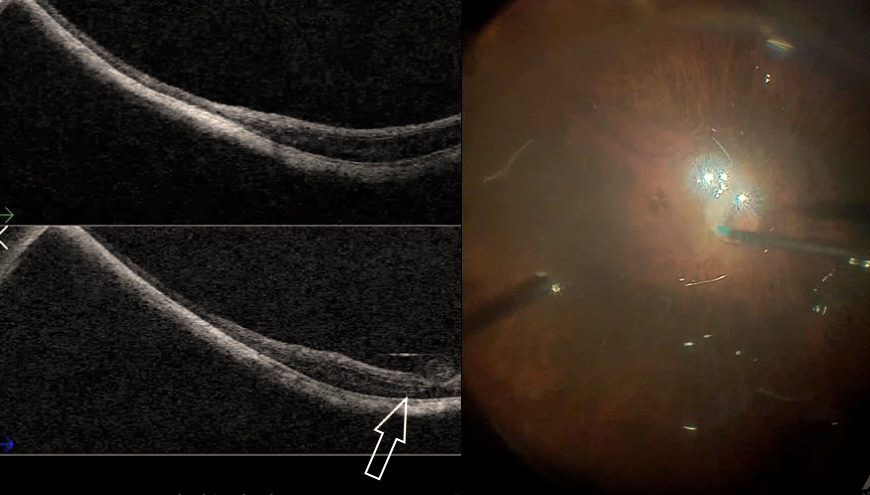
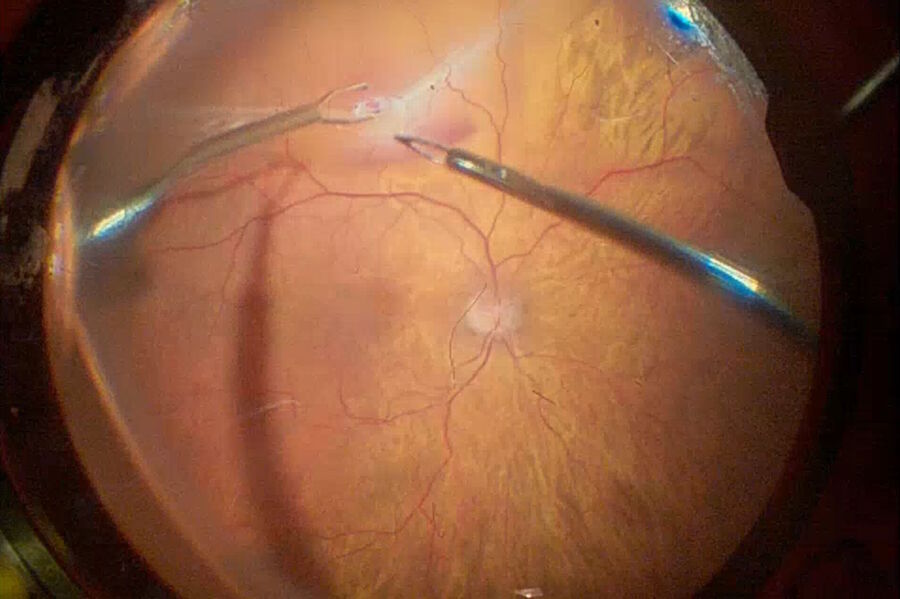
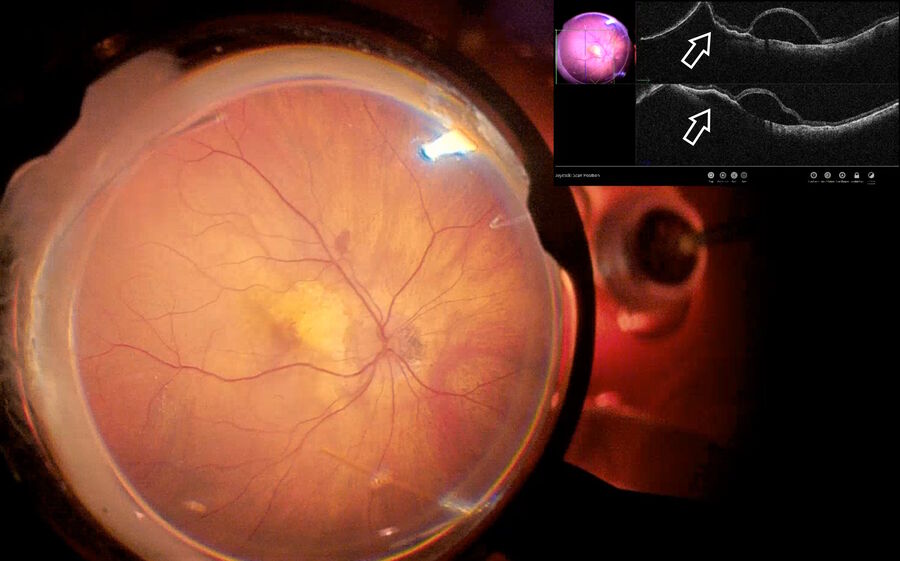
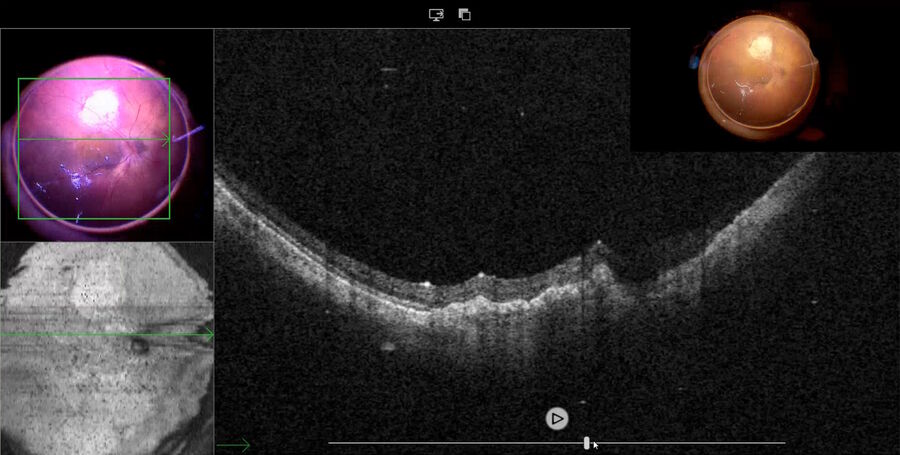
Fig. 2 depicts the case of a 52-year-old Asian female patient, presenting with high myopia, a macular hole, and macular detachment, who underwent a 23-gauge vitrectomy and internal limiting membrane (ILM) peeling. Using intraoperative OCT, we were able to visualize the presence of subretinal fluid at the beginning of the procedure. After complete ILM peeling and fluid-air exchange, persistent subretinal fluid was identified by OCT. Using an aspiration 25-gauge cannula (Grieshaber ®), the fluid was completely drained from the subretinal space (Fig. 3). As such intraoperative OCT helped to drain the subretinal fluid, while avoiding touching the retina and RPE.
2. Macular hole repair and the inverted ILM flap technique
Recently, a study has demonstrated that the inverted ILM flap technique was superior in comparison with ILM peeling.16 However, visualization of the ILM flap is difficult. Intraoperative visualization of the inverted ILM flap was made possible by OCT, which we use in our daily cases. We used it in our daily cases. Fig. 4 depicts a high myopia patient being operated with inverted ILM, while intraoperative OCT shows that the macular hole is nearly closed. Also, the ILM flap is well positioned above the fovea. Fig. 5 shows the same patient depicted in Fig. 2 and 3 with another case of inverted ILM flap technique. These images show that intraoperative OCT can be used to check the ILM positioning at the end of the procedure to help surgeons achieve improved results.
The benefits of using intraoperative OCT in macular hole repair:
- Intraoperative analysis of epiretinal membrane (to choose the optimal location to begin the peel)
- Reduced risk of iatrogenic damage in subretinal fluid aspiration during macular detachment repair (helping to avoid touching the RPE and the retina margin)
- Checking of the final positioning of the ILM flap (inverted ILM flap technique).
- Evaluation of macular hole closure (serving as a potential postoperative positioning guide)
3. Retinal detachment with subretinal fibrosis
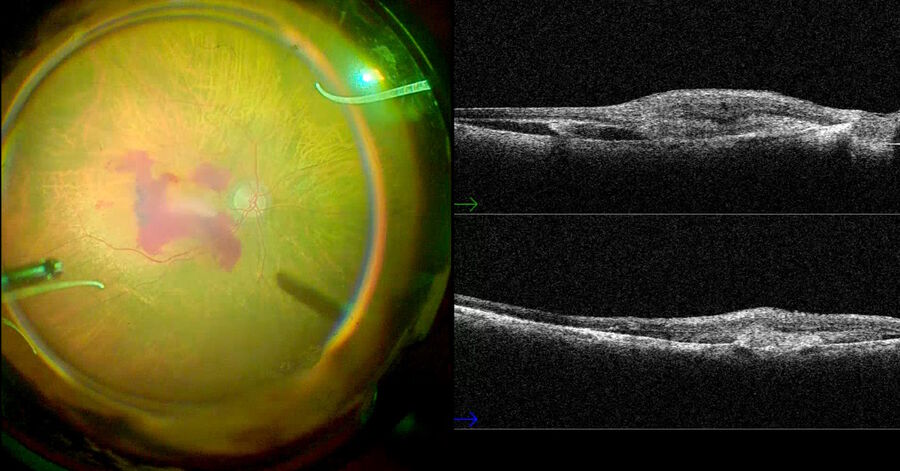
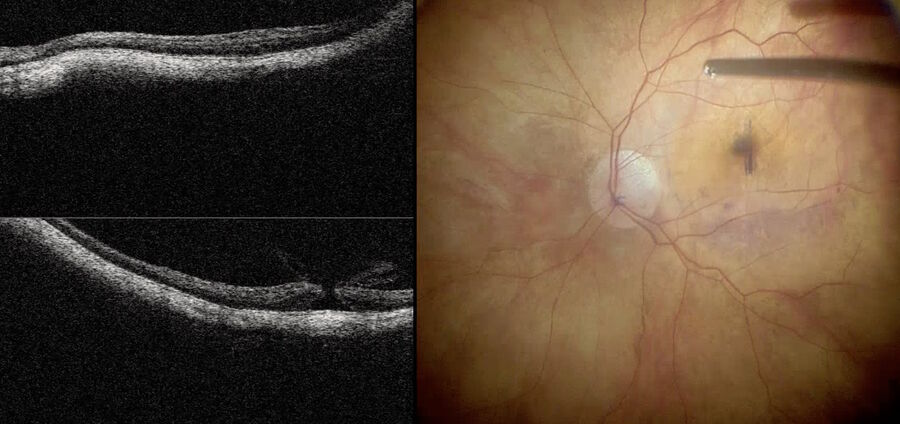
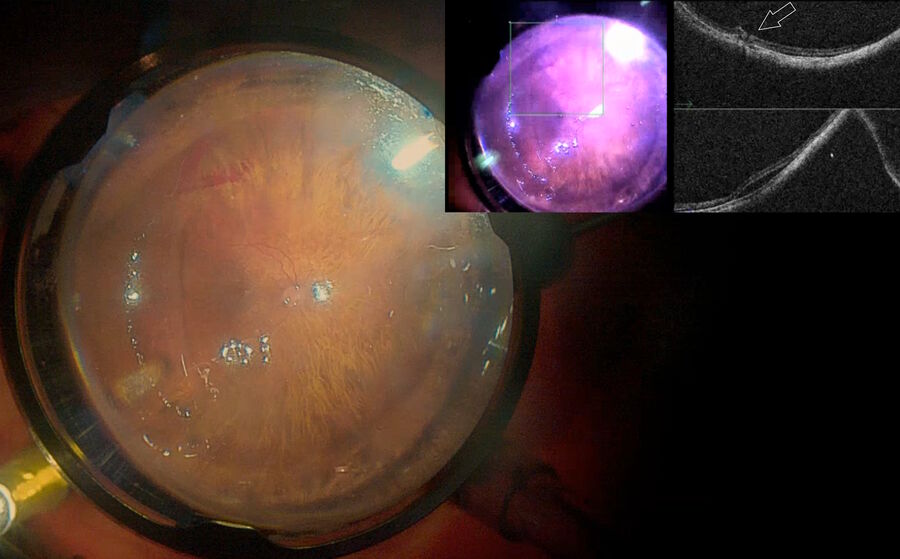
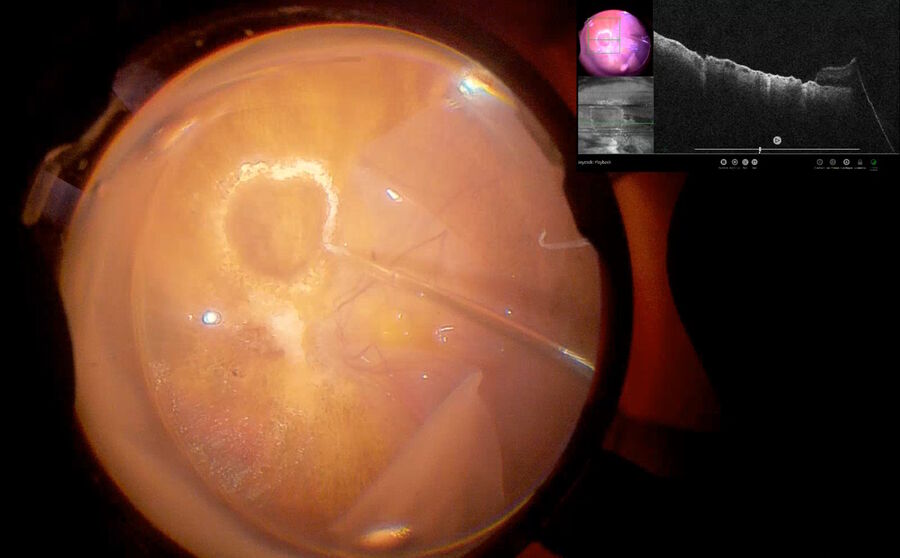
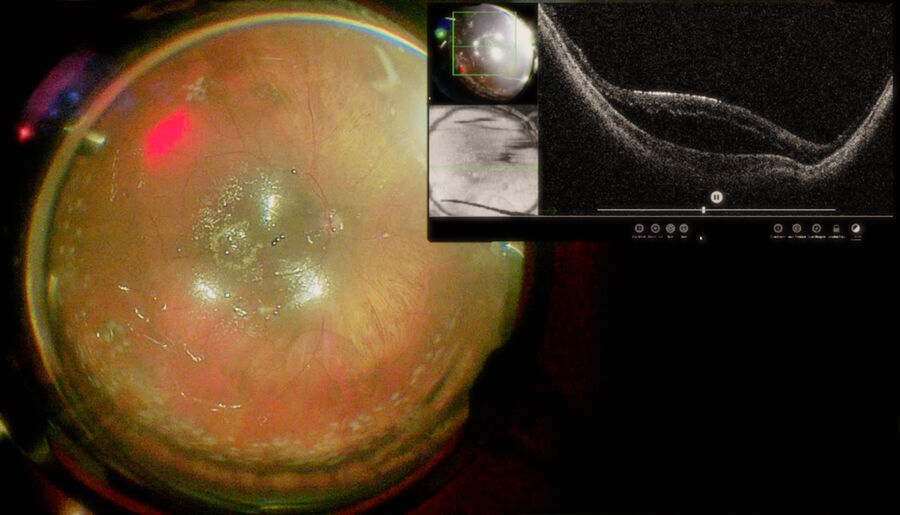
Retinal detachment complicated with proliferative vitreoretinopathy (PVR) or subretinal fibrosis has a high rate of recurrence. The complete elimination of retinal traction is critical to ensure the success of the surgery. However, in some cases it is challenging to establish whether the PVR has been removed. Moreover, it might be difficult to establish differences between PVR, and the subretinal fibrosis band. Using intraoperative OCT can offer important advantages to surgeons during these procedures. Fig. 6 shows a case of chronic inferior retinal detachment.
The PVR was removed and the perfluorocarbon liquid (PFCL) was injected into the vitreous cavity. It was possible to observe that the inferior retina had a shallow detachment. Moreover, the intraoperative OCT had confirmed that the inferior retina was not flat and showed a subretinal fibrotic band.
The PFCL was removed and an endodiathermy probe and 25-gauge forceps were used to remove the fibrosis (Fig. 7), flattening the retina. A fluid-air exchange was then performed, and a gas tamponade was used to attach the retina (Fig. 8). The use of intraoperative OCT contributed to the visualization of the subretinal band, its removal and further observation of retinal reattachment. In this case, intraoperative OCT was vital for distinguishing subretinal fibrosis from PVR and ensuring retinal reattachment.
4. Subretinal hemorrhage
Subretinal surgeries have been increasingly performed in recent years, and the injection of tissue plasminogen activator (TPA) can be facilitated by intraoperative OCT. Since the treatment for genetic conditions continues to evolve, mastering the technique of subretinal injections is crucial for the retinal surgeon.
Extensive subretinal hemorrhages may be caused by any macular condition associated with choroidal neovascularization. The most common cause is age-related macular degeneration (AMD). Other cases have been described as polypoidal choroidal vasculopathy (PCV), pathologic myopia, angioid streaks, trauma, and retinal arterial macroaneurysm.
Due to the prognsis of submacular hemorrhages, many different surgery modalities have been used in an attempt to improve visual outcomes. The result of a retrospective study suggests that pars plana vitrectomy, subretinal TPA with pneumatic displacement lead to better visual outcomes compared to the natural course of this disease [17].
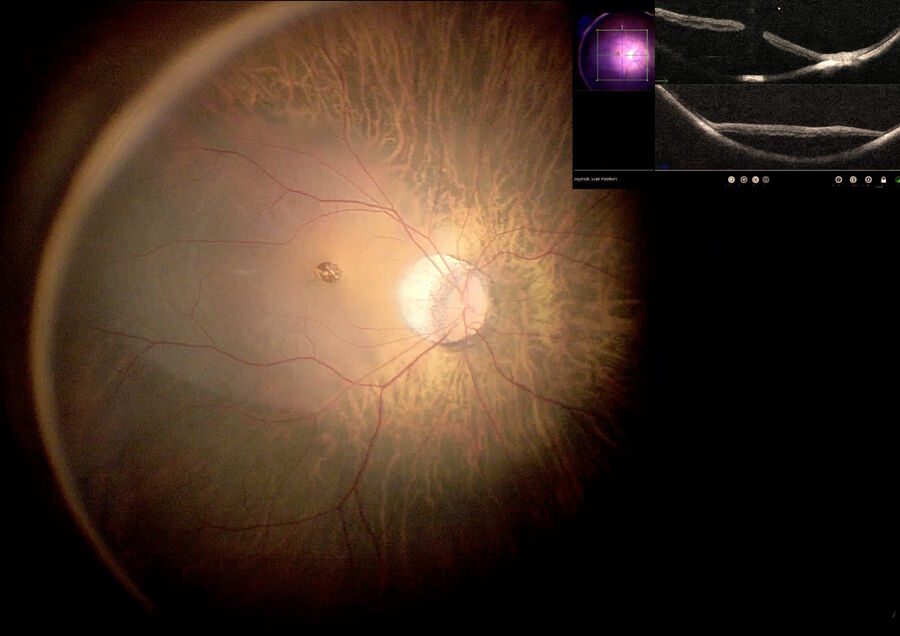
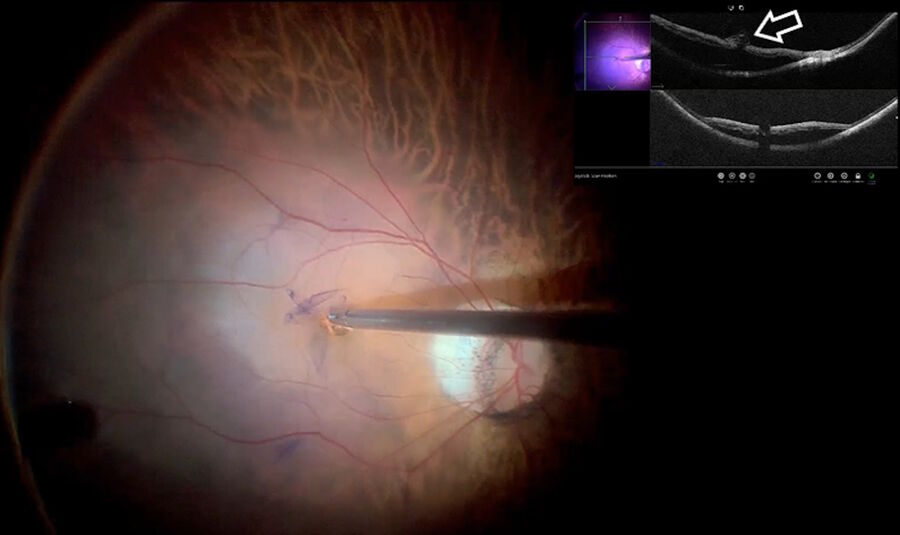
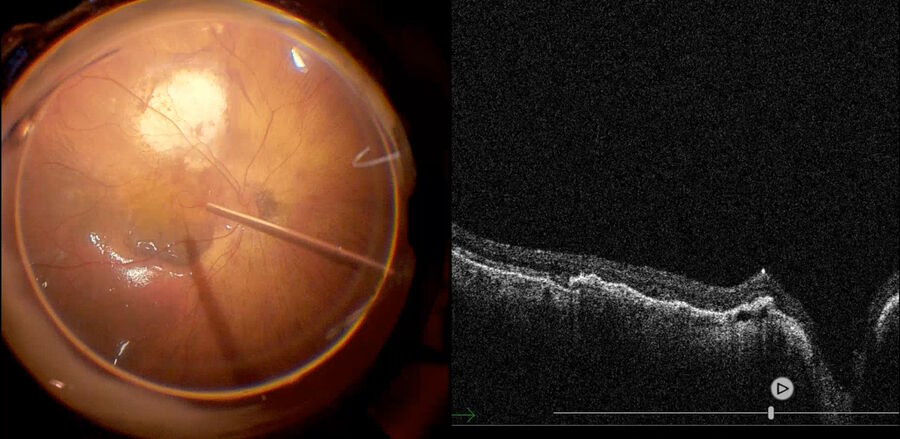
In our clinic, a case of extensive submacular hemorrhage was managed using assisted OCT (Fig. 10). Specifically, a 23-gauge pars plana vitrectomy with posterior vitreous detachment and ILM peeling was performed. A 36-gauge cannula connected to a fluid injector was inserted near the temporal vascular arcades and BSS fluid was injected to create a subretinal space. The retinal posterior pole was detached. After that, subretinal TPA with anti-VEGF was injected with a 36-gauge cannula (25ug/0,1 ml). Fluid-air exchange was performed, pneumatic displacement was then realized with C3F8 15% and the patient was instructed to maintain the head in an upright position.
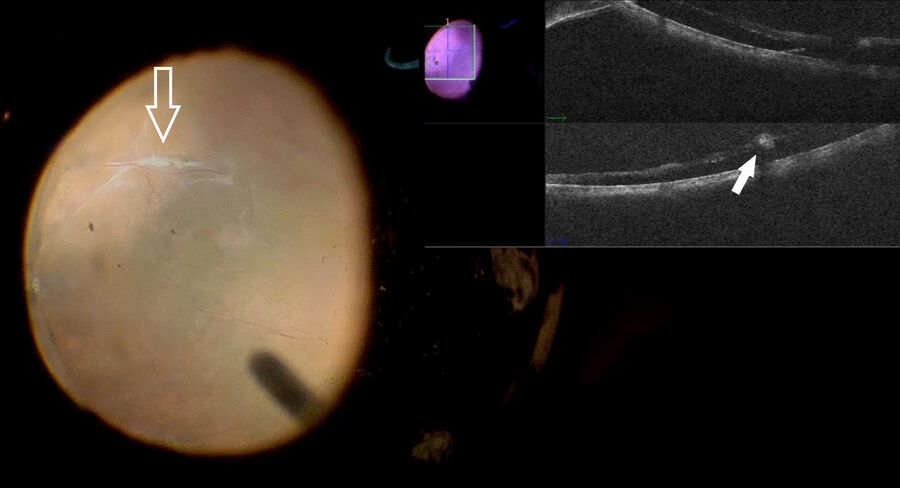
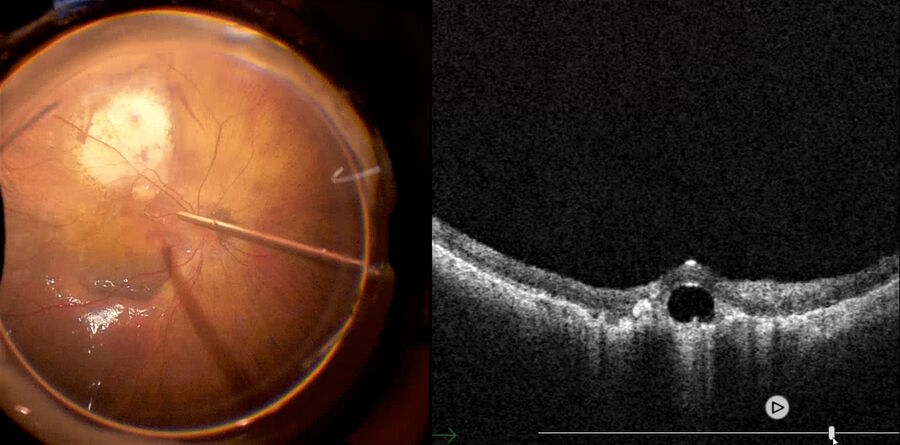
In addition, intraoperative OCT was used to observe the interaction between the TPA cannula and the retina. The subretinal positioning of the cannula was confirmed with intraoperative OCT; the fluid was safely injected, and the macula was detached (Fig. 10). At this point, a possible complication can arise during insertion of the cannula into the sub-choroidal space, as we can see in the example shown below in Fig. 11.
The wrong maneuver was promptly identified and intraoperative OCT was used to correct it (Fig. 11). Another potential complication arising after submacular injection is creation of an iatrogenic macula hole. However, OCT use allows preventing misdiagnosis of this complication.
5. Autologous RPE transplantation
RPE transplantation has been investigated as an option for the treatment of retinal damage in exudative or atrophic maculopathies such as RPE tears in age-related macular degeneration (AMD), massive subretinal hemorrhage, and geographic atrophy.18 It is an advanced technical procedure that requires accurate surgical dexterity. The additional use of intraoperative OCT can offer some benefits for RPE transplantation, since this technology provides real-time feedback about the correct positioning of the patch.
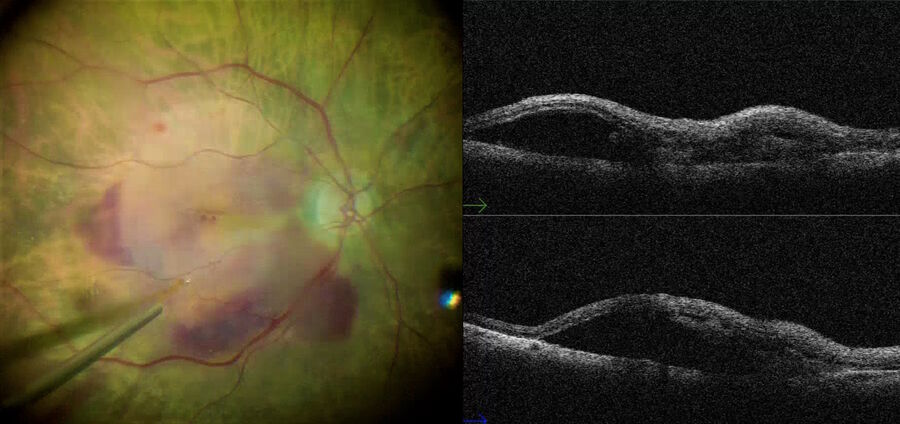
Figs. 11 to 14 depict a RPE transplantation procedure performed in an 86-year-old woman with choroidal neovascularization related to AMD. During the procedure, intraoperative OCT helped to confirm the precise positioning of the RPE transplant. Also, intraoperative OCT showed a PFCL bubble in the inferior border of the patch, with liquid removal also guided by this technology. Thus, intraoperative OCT provided real-time feedback for the surgeon, preventing further complications.
Surgical Findings
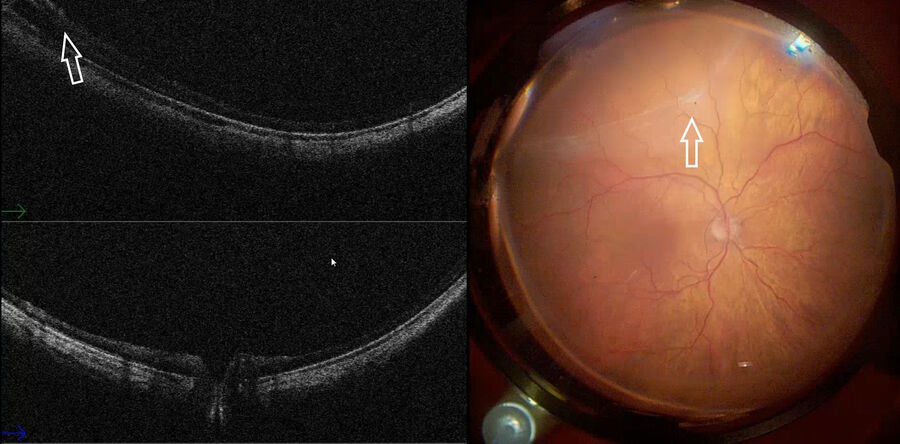
EnFocus intraoperative OCT can help to reveal important details to help surgeons during procedures. Some of these interesting features shown by intraoperative OCT have caught our attention. As we expected, forceps and probes are opaque and do not allow image formation. However, the light beam can pass through the brilliant blue dye (Membraneblue Dual ® DORC) without any restrictions. As a result, the scattering of the beam can produce clear retina images (Fig. 16).
We also observed another interesting feature during the fluid-air exchange. The intraoperative OCT image could be produced within the air, helping us determine the presence of subretinal fluid underneath (Fig. 17).
Discussion
The usefulness of intraoperative OCT in ophthalmic procedures has been evaluated in numerous studies. Its positive impact on surgical decision-making has been reported in DISCOVER and PIONEER studies (involving prospective, consecutive case series).
The PIONEER study demonstrated the feasibility of use of intraoperative OCT imaging for numerous anterior and posterior segment ophthalmic procedures in the operating room [20].
In this study, a portable spectral domain OCT (Biotigen SDOIS, Research Triangle Park, North Carolina, USA) was stabilized with a microscope-mount system. Since the OCT was not integrated into the microscope and the surgery needed to be paused to acquire images, the technology used in the PIONEER study was unable to visualize the interactions between the tissue and instruments used.
The DISCOVER study evaluated the utility of intraoperative OCT and assessed surgical outcomes in patients with retinal detachment (RD) [19]. The study involved the use of the RESCAN 700 microscope-integrated OCT (Carl Zeiss Meditec, Inc, Oberkochen, Germany), EnFocus intraoperative OCT (Biotigen/Leica Microsystems, Wetzlar, Germany) and Cole Eye Institute's microscope-integrated intraoperative OCT (Cleveland Clinic Foundation, Cleveland, Ohio). In terms of RD repairs, the study points out the potential benefits of OCT in treatment of complex pathologies, such as PVR, panuveitis, giant retinal tears and recurrent retinal detachment. Specifically, intraoperative OCT was demonstrated to provide useful feedback in 50% of complex cases, and in 22% of regular cases (non-complicated RD). The study also showed that in 12% of cases, intraoperative OCT data directly altered surgical decision making. Despite the lack of a control group, the study showed a reoperation rate of 25% in complex cases, which closely corresponds the overall reported ranges of 13-30% [19].
In our service, intraoperative OCT was used in a variety of surgical cases and allowed us to discover new insights in retinal surgery. In addition, the use of intraoperative OCT also provided a better understanding of retina structures over the course of the surgery. For example, retinal areas under suspicion of containing subretinal fluid after fluid-air exchange could be reviewed, helping to improve the surgeon’s confidence during the procedure. In addition, intraoperative OCT was also able to help the surgeon ensure the correct positioning of the inverted ILM flap, since it is not possible to detect the integrity of the ILM flap intraoperatively through any other means. Another example of the benefits of intraoperative OCT use is improved intraoperative visualization of macular hole closure. A recently published study has demonstrated that intraoperative OCT-based identification of macular hole closure could serve as a safe and helpful tool when prescribing short-term postoperative prone posturing [12].
The combination of intraoperative OCT with a 3D-assisted display system has also brought forth new possibilities for ophthalmic surgery. Through a simple connection with an HDMI cable, the image generated by EnFocus can be projected (using picture-in-picture) on the same 3D screen used by the surgeon during surgery. This makes it possible to maintain optimal ergonomics (heads-up), while reducing intraoperative time to analyze the images (since there is no need to look at another screen). Moreover, this technology allows to increase the size of the image (splitting the screen/zoom in and out) and assisting the surgeon in the decision-making process.
Limitations of Former Intraop. OCT Generations
The integration of previous generations of intraoperative OCT technology into the surgical workflow involved some challenges, and the help of an imaging assistant was often needed to assist the surgeon in handling the intraoperative OCT modality during surgery. For example, surgeons required assistance during procedures when it was necessary to change a scan pattern or optimize image quality of live OCT images.
Despite the possibility of using a preprogrammed footswitch to independently control movements in the X-Y-Z axis, capture still images, and acquire scans, surgeons relied on their assistants during the surgery to support them in efficient operation of the OCT.
Recent advances in the integration of OCT into the surgical workflow have succeeded in closing this gap. The next-generation EnFocus intraoperative OCT built-into the Proveo 8 surgical microscope (Leica Microsystems) provides the surgeon with maximum freedom. Using this cutting-edge technology, surgeons can work smoothly and independently. In addition, there is no more need to invest time and effort in optimizing and aligning OCT imaging during live procedures. The new automatic image optimization and tracking of the EnFocus OCT help to assure that surgeons will always have consistent, optimized OCT imaging available whenever it is needed.
Furthermore, using a technician to operate OCT during procedures is not required anymore, as surgeons can switch from the microscope view to an OCT view effortlessly, with a tap on the footswitch or on the handgrip. The full integration of OCT into the Proveo 8 microscope also facilitates recording of procedures, which can be controlled with the microscope footswitch, handgrip or even via touchscreen control of the microscope's monitor. The latter can be used to facilitate touch-friendly gestures on the user-interface screen, such as manual scrolling and using the two-finger pinch to resize images, as well as pointing to specific locations, moving and rotating OCT scans.
Conclusion
Retinal surgery continues to evolve, while surgeons continuously strive to further optimize and develop their surgical skills and techniques to improve clinical outcomes. With improved integration of intraoperative OCT modalities into the surgical workflow, the microscopic view becomes supplemented with bright and sharp images of previously hidden subsurface details, which enable surgeons to better understand ocular pathology. This novel technology will further transform retina surgery. Furthermore, intraoperative imaging in real-time provides a tremendous advantage for surgeons, as it allows them to observe the way retinal tissue reacts to surgical maneuvers. Moreover, intraoperative OCT provides surgeons with high-resolution images and immediate confirmation of surgical maneuvers, allowing observation of tissue changes at every step of the procedure. This allows surgeons to gain confidence and improve patient outcomes.
The combination of intraoperative OCT imaging with a 3D visualization system brings further ergonomic benefits to the surgical workflow, while minimizing interruptions and capturing quality OCT images. In our surgical practice, we display both images together on a single monitor. Moreover, intraoperative OCT is especially useful in cases with pre-retinal inflammatory precipitates (PIP), as this technology visually aids in performing sensitive maneuvers that require a high level of precision, while helping to avoid damaging retinal tissue.
The use of intraoperative OCT during complex eye surgery, and especially in retina surgery, opens up new possibilities for improved clinical treatment and potentially improving postoperative results.
The clinical utility of intraoperative OCT technology will become more evident as more prospective clinical trials are conducted, which will provide additional evidence of its efficacy and the associated benefits for patient outcomes.
The authors
Dr. André Maia, Dr. Ricardo Okada Nakaghi, Dr. Gabriel Costa de Andrade, Dr. Francisco Rosa Stefanini, Dr. Hermano Assis Filho, Caio Franco da Silveira, Dr. Rafael Reis Pereira, Dr. Lucas Mares Guia Ribeiro, Retina Clinic, São Paulo, Brazil
The statements of the healthcare professionals made in this white paper reflect only their personal opinions and experiences. These statements do not necessarily reflect the opinion of any institution with whom they are affiliated.