The Solution
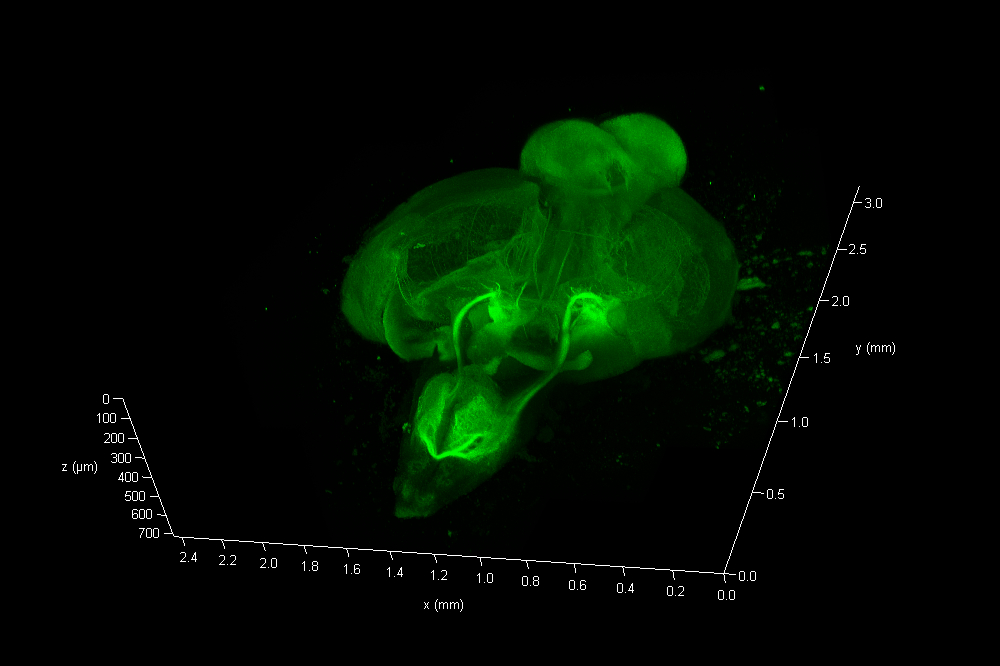
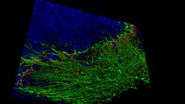
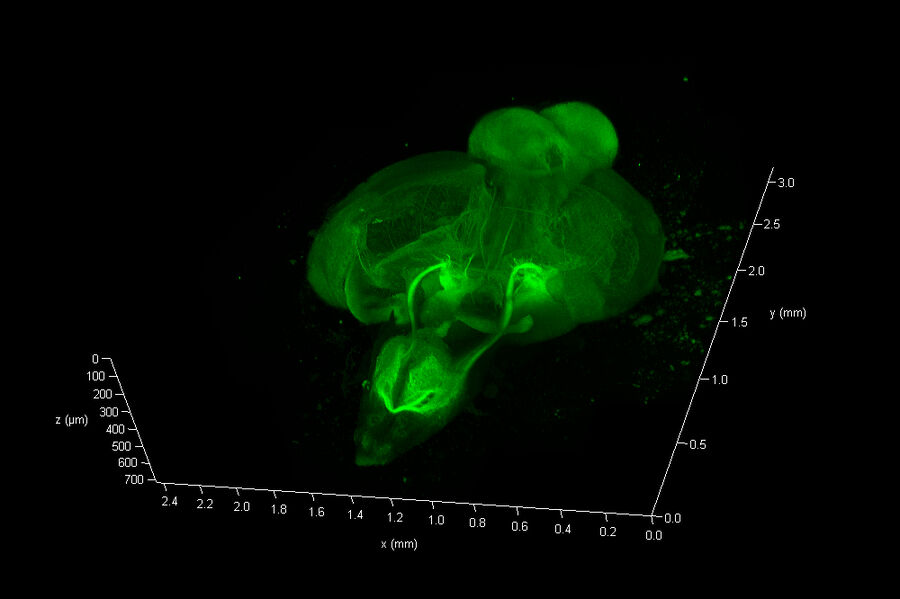
The Link Lab at the Medical College of Wisconsin studies ocular development and disease, focusing on zebrafish models for retinal neurogenesis. Eric Clark, a Ph.D candidate in the Link Lab, sought to create a full-volume 3D image of a whole zebrafish brain approximately 750 microns in depth, and spanning an area approximate 3 millimeters in diameter. To image the nervous system, the animal was first transfected with a construct expressing eGFP behind a pan-neuronal promoter, resulting in a ubiquitous green fluorescence throughout neurons in the CNS.
Sample clearing and objective lens
To reduce scattering caused by changes in refractive index(RI), the dissected CNS sample was cleared via passive clarity. A custom Histodenz based refractive index matching solution was used to maintain consistent RI throughout the optical path. The prepared organ was imaged using a Leica HC FLUOTAR L 25x/1.00 IMM objective lens, that offers 250 nm optical resolution in a field of ca 1mm x 1mm at up to 6 mm free working distance. This objective is equipped with a motorized correction (motCORR) collar, which can be adjusted to compensate for the effects of RI variance at depth.
Video animation of eGFP stained zebrafish
Collection of high resolved large field
To collect data from throughout the brain, Eric utilized the tile-scan functionality provided by the LAS X NAVIGATOR acquisition software. Navigator allows a user to simply record optical image stacks in a large array of stitched microscopic fields and fuse them to a single 3D data set, automatically balancing intensity variations during the stitching and acquisition processes. The resulting data can be rendered via 3D visualization software including the 3D viewer within LAS X (see movie fig 01).
The final data consisted of 286 image planes, each 5675 x 7836 pixels. A total of 12.7 Giga-Voxels.
Photon efficiency and sample protection
A precondition to collect such immense data sets from a fluorescent sample is a very sensitive detection system, necessary to reach a high signal to noise ratio in a comparably short time frame and with minimal photo-bleaching. To accomplish this, imaging was performed using a confocal microscope equipped with Leica’s virtually noise free Hybrid Detectors (HyD), an acousto optical beam splitter [1] (AOBS), and a prism based spectral detector [2] (SP-detector) for filter-free spectral separation and freely tunable bandwidth specifications.
The User’s Voice
“The Leica Clarity objective with motCORR provided the ability to match the refractive index of the sample clearing/mounting media, and offered the extended working distance and light gathering capability to image this sensitive sample at depth.”
Eric Clark, Ph.D. candidate, Medical College of Wisconsin. Milwaukee, Wisconsin (USA)