Development and Derisking of CRISPR Therapies for Rare Diseases
How AI-Microscopy enables rapid, precise analysis to fast-track gene editing cures for critically ill children with rare genetic immune disorders
This on-demand presentation by Dr. Fyodor Urnov and Dr. Sadik Kassim, originally delivered at ASGCT 2025, focused on a critical challenge in genetic medicine: how to scale CRISPR therapies from single-disease solutions to a platform approach, particularly for rare pediatric genetic disorders.
Dr. Urnov showcased work at the Innovative Genomics Institute led by Dr. Matthew Kan as part of the IGI-Danaher Beacon for CRISPR Cures. This particular effort relied on the combined power of a Leica Microsystems microscope and Aivia AI software to enable a first-of-its-kind, high-throughput, automated, and quantitative analysis of CRISPR editing outcomes in cell-based assays where current methods fell short.
The uniquely enabling role of Leica Microsystems in gene editing therapy development
The THUNDER Imager Live Cell & 3D Assay and Aivia AI-powered image analysis software provided foundational tools that translated imaging into actionable CRISPR insights—driving faster, scalable, and more precise development of genetic therapies. The combination of advanced microscopy and AI is a mission-critical tool in a field where days can make a difference for the patients’ outcome.
- Rapidly and quantitively assess off-target genome editor activity in clinically relevant primary human cells without relying on sequencing.
- Rapidly identify the most specific and effective guide RNA candidates in real-world timelines.
- Integrate advanced AI image processing in preclinical decision-making.
Microscopy-based DNA damage quantification
The microscopes from Leica Microsystems were part of a broader hardware ecosystem from Danaher, with Beckman Coulter Life Sciences, Molecular Devices, etc., integrated into a CRISPR-based therapy workflow. Together, these tools will be crucial for cell phenotyping, potency assays, and manufacturing quality control.
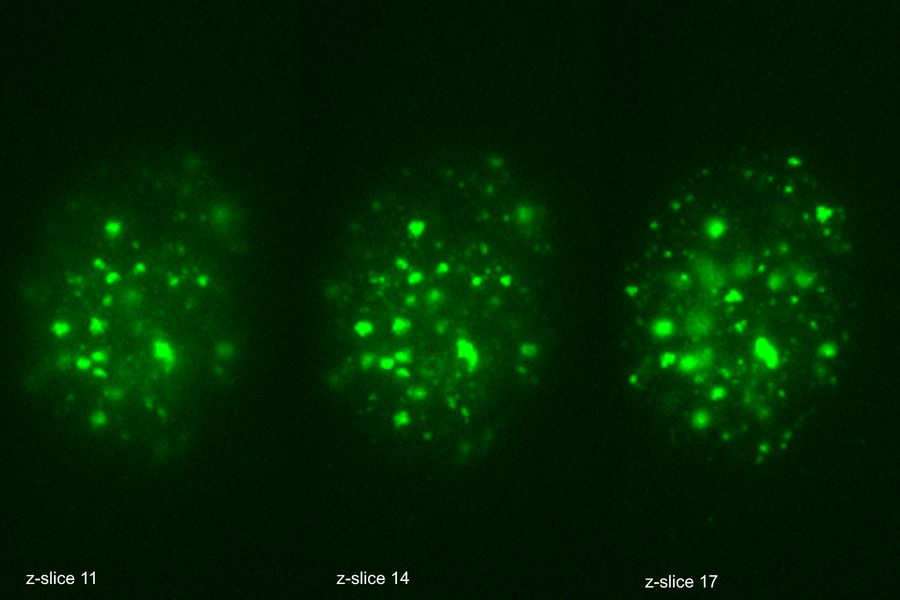
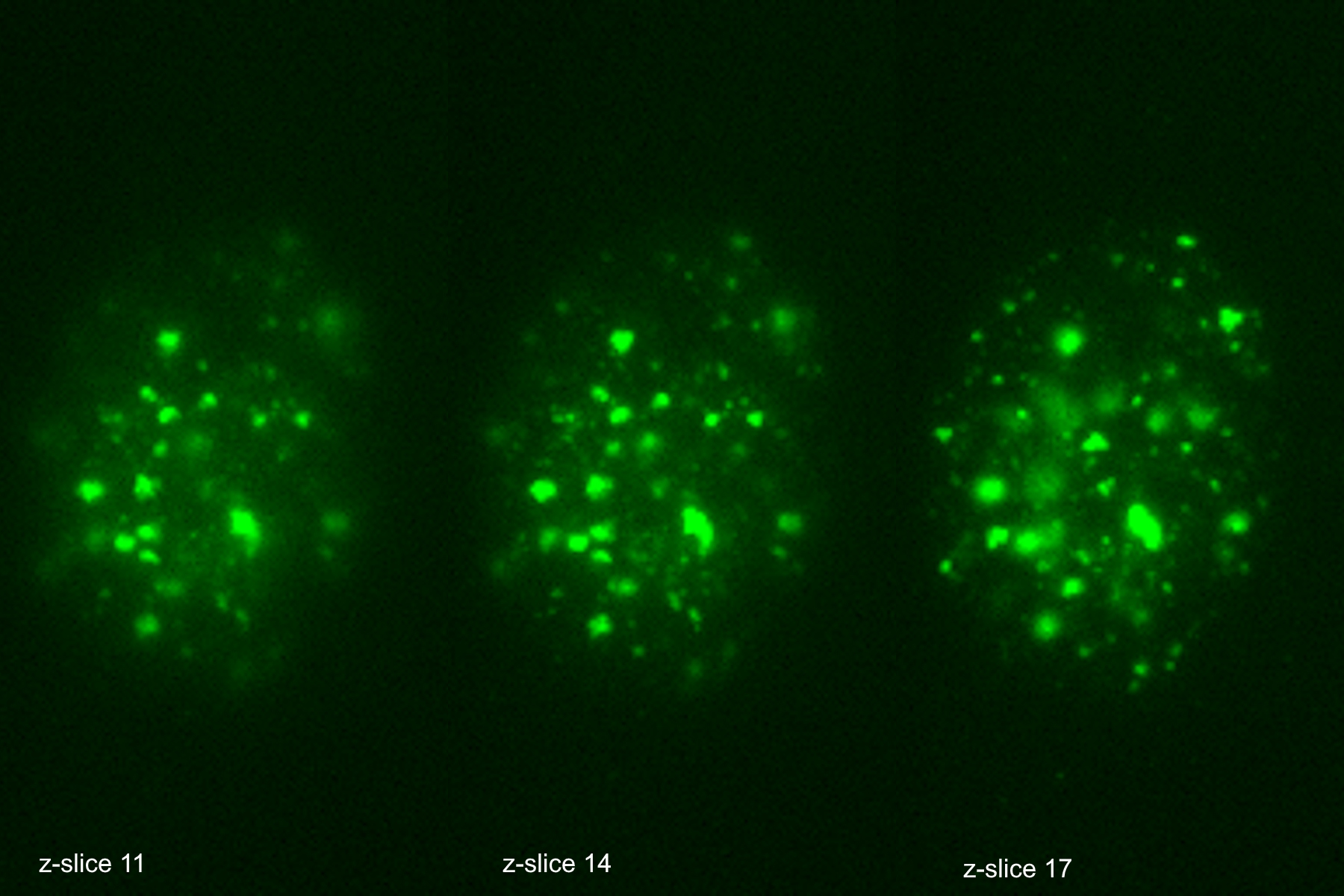
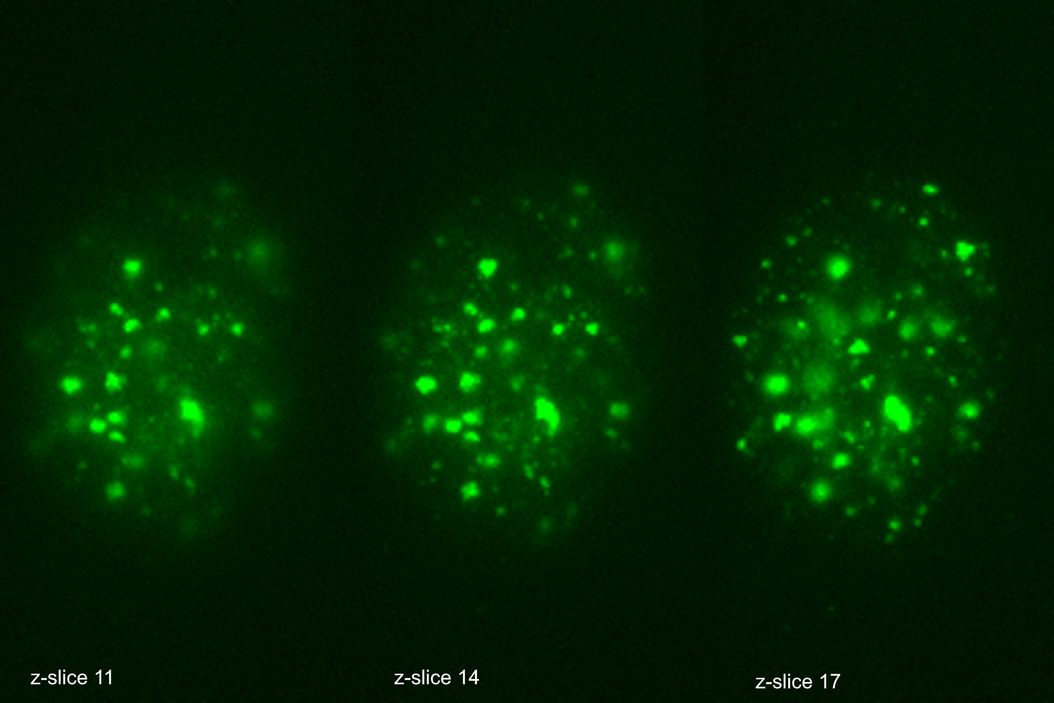
The THUNDER Imager was used in tandem with Aivia to analyze DNA double-strand break (DSB) foci in edited human T cells. Traditional DSB imaging is complex due to the three-dimensional distribution of breaks within the nucleus. The THUNDER Imager enables high resolution wide-field imaging, allowing for detailed images of tens of thousands of cells. The Extended Depth of Field (EDF) algorithm from Leica Microsystems and Aivia “flattened” the images computationally, allowing for reliable 2D quantification and substantially decrease computational complexity. The AI algorithm was trained to call DSB positive staining into countable discrete objects, enabling researchers to quantify DNA damage across 10,000+ cells per sample in under four days.
Specificity assays
By comparing guide RNAs available for the targeted gene using the THUNDER Imager plus Aivia complete analysis workflow, researchers were able to quickly downselect to the most specific CRISPR guides, reducing regulatory risk and streamlining therapeutic development.