Organoids
Organoids are 3D cell culture models that provide a more realistic representation of how cells interact and function in three dimensions. Unlike traditional 2D cultures, these models can, to a certain extent, replicate the physiology of various organs in vitro. Organoids develop over time to reach sizes of up to several millimeters, and light scattering makes imaging deeper layers with sufficient single-cell resolution increasingly difficult. The dual-view detection system of the Viventis Deep helps address this limitation by improving image quality throughout the entire sample volume.
Brain organoid
40-hour time-lapse of a human brain organoid generated from induced pluripotent stem cell (iPSC) lines expressing Lamin (LAMB1)-RFP (shown in green) and Actin (ACTB)-GFP (shown in magenta). The video shows a maximum intensity projection along the z-axis. Unguided brain organoids derived from PSCs can develop self-organized, regionalized domains that remarkably resemble parts of the human brain. This makes them an incredibly powerful in vitro model to studying brain development. The time-lapse video is shown on the left and a 3D representation of the first time point is displayed on the right. The time interval between frames is 30 minutes. Courtesy of Akanksha Jain (Treutlein Lab, ETH-DBSSE, Basel, Switzerland). Jain and colleagues obtained similar results using the Viventis LS1 Live microscope, filming brain organoids up to 188 hours. Jain, A., Gut, G., Sanchis-Calleja, F. et al. Morphodynamics of human early brain organoid development. Nature (2025). https://doi.org/10.1038/s41586-025-09151-3
Liver organoids
50-hour time-lapse of two murine liver organoids. The video shows a maximum intensity projection along the z-axis. Nuclei are marked with H2B-mCherry (magenta) and cell membranes with mg-GFP (cyan). The long-term imaging reveals repeating cycles of inflation and deflation of the organoids. Moos, F., Suppinger, S., de Medeiros, G. et al. Open-top multisample dual-view light-sheet microscope for live imaging of large multicellular systems. Nat Methods 21, 798–803 (2024). https://doi.org/10.1038/s41592-024-02213-w
3D representation of a murine liver organoid. Nuclei are marked with H2B-mCherry (magenta) and cell membranes with mg-GFP (cyan). 3D reconstruction and nuclei segmentation were performed using the AI image analysis software Aivia. The detailed volumetric acquisition enabled by the dual-view detection of Viventis Deep allowed for the accurate segmentation of nuclei of the entire sample. Moos, F., Suppinger, S., de Medeiros, G. et al. Open-top multisample dual-view light-sheet microscope for live imaging of large multicellular systems. Nat Methods 21, 798–803 (2024). https://doi.org/10.1038/s41592-024-02213-w
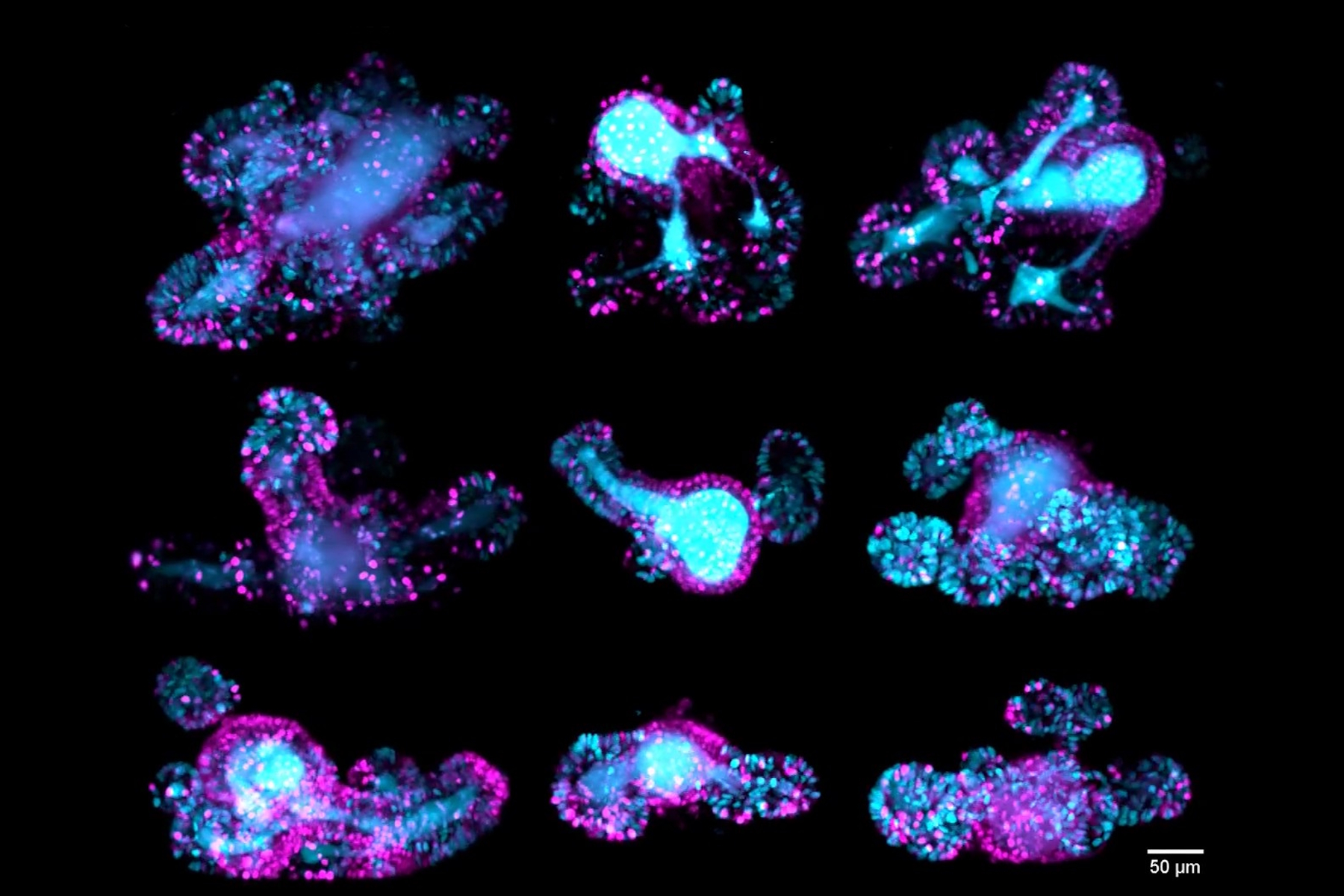
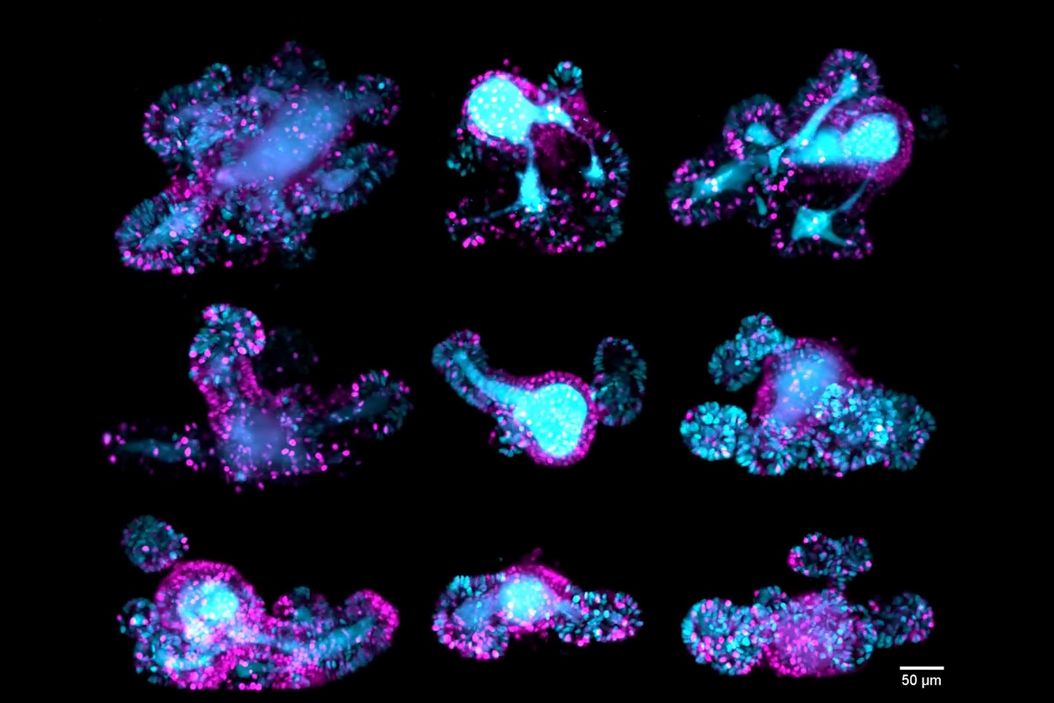
Intestinal organoids
Z-stack of a mouse intestinal organoid expressing the cell cycle reporter FUCCI2 (hGem-mVenus and hCdt1-mCherry). Dividing cells of the crypts are shown in cyan and resting cells of the villi are shown in magenta. The video compares the image quality of detection objective 1 (left), detection objective 2 (middle) and the fused data (right) The dual view detection of Viventis Deep and fusion of the two Z-stacks (260 µm) enabled optimal quality throughout the entire volume of the intestinal organoid. Moos, F., Suppinger, S., de Medeiros, G. et al. Open-top multisample dual-view light-sheet microscope for live imaging of large multicellular systems. Nat Methods 21, 798–803 (2024). https://doi.org/10.1038/s41592-024-02213-w
67-hour, multi-position time-lapse of mouse intestinal organoids expressing the cell cycle reporter FUCCI2 (hGem-mVenus and hCdt1-mCherry). Maximum-intensity projection along the z-axis. The custom-developed sample holder by Viventis enabled researchers to film multiple organoids in parallel and follow their cell cycle dynamics. The dual-detection capability of Viventis Deep allowed the simultaneous imaging of both crypts (dividing cells labeled in cyan) and villi (resting cells labeled in magenta) with single-cell resolution within a depth of almost 400 µm. Moos, F., Suppinger, S., de Medeiros, G. et al. Open-top multisample dual-view light-sheet microscope for live imaging of large multicellular systems. Nat Methods 21, 798–803 (2024). https://doi.org/10.1038/s41592-024-02213-w
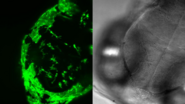
Cancer organoid
137-hour time-lapse of a human colon cancer organoid. A 3D representation was generated using the AI image analysis software Aivia. Nuclei expressing H2B-mNeon are shown in cyan. The growth evolution of the cancer organoid was filmed for almost 6 days. The time interval between frames was 30 minutes. The video also highlights the optimal quality that can be obtained throughout the entire volume of dense 3D scattering samples with the dual-view detection of Viventis Deep.
Moos, F., Suppinger, S., de Medeiros, G. et al. Open-top multisample dual-view light-sheet microscope for live imaging of large multicellular systems. Nat Methods 21, 798–803 (2024). https://doi.org/10.1038/s41592-024-02213-w
15-hour time-lapse of a co-culture between a mammary gland organoid and macrophages. Maximum-intensity projection along the z-axis. Macrophages (shown in hot orange) rapidly move within the substrate and interact via their protrusions with mammary gland cells (shown in hot cyan) as observed at the periphery of the organoid. Time scale is hours:minutes, and the time interval between frames is 30 minutes. Courtesy of Aurelie Chiche, Li lab, Pasteur Institute, Paris, France.
Embryo development
Developing embryos provide a dynamic model to study early developmental processes, such as cell differentiation and tissue formation. They also help scientists understand congenital disorders and improve regenerative medicine by revealing how complex organisms form from a single cell. Because embryos are particularly sensitive to light exposure during imaging and require regular medium exchange for proper in vitro development, the Viventis Deep systems, thanks to its open-top sample holder, provides an excellent solution for long-term 3D imaging of developing embryos.
Mouse embryo
40-hour time-lapse of an early mouse embryo. The spectacular time-lapse video captures the moment when cardiac cells (shown in hot cyan) in a mouse embryo begin to organize during early development. The open-top design of the Viventis LS1 Live enabled the researchers to exchange media during the time-lapse experiments. This was crucial for maintaining optimal physiological conditions, allowing embryos to develop normally from E6.5 for up to 40 hours, at which point the formation of a heart tube could be observed. Courtesy of Kenzo Ivanovitch, UCL, London, UK. EMBO Journal www.embopress.org/doi/full/10.1038/s44318-025-00441-0
Zebrafish embryos
5-hour time-lapse of a zebrafish embryo at 3 days post fertilization. Maximum-intensity projection along the z-axis. Microglia cell membrane (mpeg1:Gal4; UAS:lyn-tagRFPT) is shown in green. The video on the left shows microglia cells moving within the optic tectum. As observed in the video, microglia cells have highly dynamic extensions that they use to scan the brain in search of dying neurons to be removed. On the right, the brightfield channel is shown. Courtesy of Francesca Peri, University of Zurich, Switzerland.
9-hour time-lapse of a zebrafish embryo. Maximum-intensity projection along the z-axis. Nuclei are marked with H2B-eGFP (grey). The video shows the process of epiboly during gastrulation observed from the two opposing detections of Viventis Deep (Detection 1 on the left and Detection 2 on the right). The time scale is hours:minutes. Courtesy of Oates Lab, EPFL, Lausanne, Switzerland.
Aquatic organisms
Many aquatic species share key genetic and developmental pathways with higher vertebrates, making them valuable for studying gene function, disease mechanisms, and early development. Their externally developing embryos and transparent tissues allow scientists to observe biological processes in real time, providing insights that are often conserved across species. These studies can greatly benefit from the dual-illumination and dual-view detection capabilities of the Viventis Deep light-sheet microscope, especially with its open-top configuration designed for long-term imaging.
Hydrae
67-hour time-lapse of three examples of Hydra regeneration. Maximum-intensity projection along the z-axis. Animals express an ectoderm-reporter (ecto [β-act-RFP]) shown in grey. Spheroids were obtained by cutting adult Hydra and were filmed for almost 3 days to observe the regeneration of the body axis. Hydra is a small freshwater cnidarian polyp, and it serves as a fascinating model system with an incredible capacity for regeneration. Moos, F., Suppinger, S., de Medeiros, G. et al. Open-top multisample dual-view light-sheet microscope for live imaging of large multicellular systems. Nat Methods 21, 798–803 (2024). https://doi.org/10.1038/s41592-024-02213-w
Z-stack of Hydra polyp expressing an ectoderm-reporter (ecto [β-act-RFP]) shown in grey. Scan through the 460-micron z-stack comparing the image quality of detection objectives 1 (left) and 2 (middle) and the fused data (right) of Hydra. In Viventis Deep, the dual-view detection allows large multicellular systems to be acquired with optimal quality throughout the entire volume. Moos, F., Suppinger, S., de Medeiros, G. et al. Open-top multisample dual-view light-sheet microscope for live imaging of large multicellular systems. Nat Methods 21, 798–803 (2024). https://doi.org/10.1038/s41592-024-02213-w
Ascidian embryo
Three-hour time-lapse of an early-stage Ascidian blastula. The 3D representation was generated using the AI image analysis software Aivia. To label cell membranes (shown in cyan), PH::Tomato mRNA was injected into the egg. The researchers aimed to acquire complete 4D (3D + time) datasets of embryos for mesh reconstruction. These meshes are used to measure angles and assess relative tension, enabling the creation of spatiotemporal biomechanical maps. The dual-view detection system of the Viventis Deep was instrumental in achieving high-quality 3D reconstructions of Ascidian embryos across the entire specimen volume. The time interval between frames was 2 minutes. Courtesy of Daniel Gonzalez Suarez, LBDV, Villefranche sur Mer, France.
Sea urchin juvenile
Sea urchin juvenile at 1-week post-metamorphosis. The 3D representation was created with AI image analysis software Aivia. Localization of gene expression by HCR in situ hybridization of two circadian clock genes, shown in cyan and purple. Nuclei are shown in grey. The dual-view detection of Viventis Deep allowed the researchers to clearly reconstruct the internal anatomy of the specimen and after in situ hybridization, to localize the transcript signals. Courtesy of Rossella Annunziata, Stazione Zoologica Anton Dohrn, Naples, Italy.
Plants
Long-term 3D imaging is highly beneficial in plant biology, as it allows researchers to observe dynamic processes in real time within living tissues. The open-top design of the Viventis Deep system, along with its custom-developed sample holder, enables easy mounting of plant seedlings and allows them to grow vertically.
Arabidopsis thaliana root
This 20-hour time-lapse captures the growth of an Arabidopsis thaliana root at six days post-germination, visualized using a maximum-intensity projection along the z-axis. The left panel displays plasma membranes labeled with UBQ10::RCI2A-TdTomato (visualized in hot cyan), while the right panel shows the corresponding brightfield channel. A three-day-old seedling was transferred to a Viventis Deep sample well, and imaging began three days later. During the time-lapse, the root exhibited vertical growth of nearly 1 mm. Shedding of root cap cells is also visible. To maintain the root within the field of view, the automated object tracking module integrated into the Viventis software was employed throughout the imaging process. The time scale is presented in hours:minutes time interval between frames is 30 minutes. Courtesy of Jia Pang, Geldner group, University of Lausanne, Switzerland.