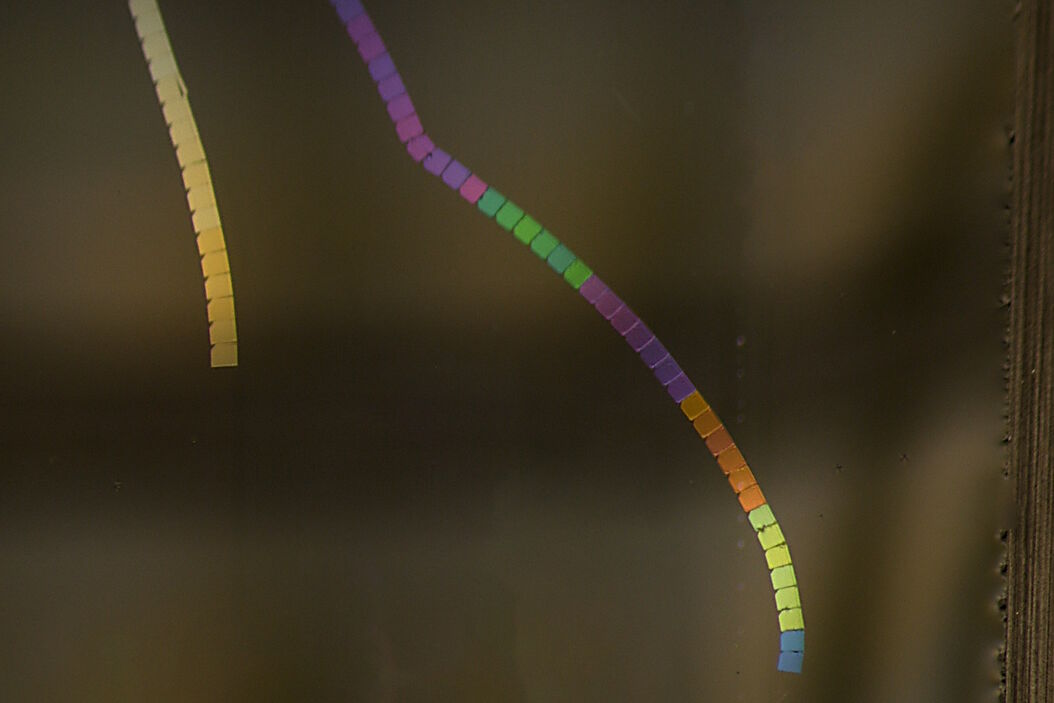Introduction
Ultramicrotomy, a technique integral to electron microscopy, facilitates the preparation of ultra-thin sections for high-resolution imaging and analysis. Due to its precision and versatility to section samples at room and cryogenic temperatures, ultramicrotomy has become an indispensable research tool in life and material sciences [1-6]. Ultramicrotomy enables scientists to control the thickness of sections cut from a sample with a high degree of accuracy. This is essential for producing sections that satisfy the requirements for imaging in a scanning or transmission electron microscopy (SEM and TEM, respectively) [1]. Typical ranges for sections thickness are 50-300 nm for TEM, while SEM array tomography typically requires sections below 50 nm [2]. The quality of sections produced through ultramicrotomy profoundly influences the reliability and interpretability of experimental results across diverse scientific disciplines. Ensuring consistent section quality is thus imperative for researchers seeking accurate data and meaningful insights [1-6].
SEM and TEM enable high-resolution imaging of biological and material structures at both cryogenic and room temperature conditions [5]. These electron microscopy (EM) techniques provide a means to observe the structural details of samples beyond the resolution limits of light microscopy methods [5]. Regardless of the specific research goals, consistent and reliable section thickness is critical for imaging and analyzing multiple samples under identical conditions [1]. Therefore, ultramicrotomy is the method of choice to prepare sections of samples that allow for successful downstream workflow steps [3].
Challenges
While achieving consistent section thickness is crucial for obtaining high-quality results in downstream experiments [1], ultramicrotomy presents several challenges that can compromise section quality.
Common problems encountered in different steps of the preparation process include insufficient clamping of the knife or specimen, external influences like building vibrations, and defective ultramicrotome setup [8]. Achieving uniform sections free of artifacts requires proper embedding, fixation, dehydration, polymerization, and careful consideration of specimen composition, knife adjustment, workplace cleanliness, and minimum section thicknesses [9]. Deviating from standard procedures can introduce new challenges like section contamination and cutting artifacts [9]. Moreover, publication bias may overlook problems and mistakes associated with new methods and instruments [8]. To ensure the quality of specimen blocks, discussing common sectioning artifacts and addressing errors in specimen preparation, knife-edge quality, cutting method, section spreading, grid collection, staining, and instrument cleanliness is essential [9].
The other challenges frequently encountered in maintaining section quality are related to the inherent variability in specimen properties, including hardness, elasticity, and composition. Specimens with uneven densities or heterogeneous structures may yield sections of inconsistent thickness or suffer from distortion during cutting. Moreover, ultrathin sections are susceptible to damage from artifacts such as wrinkles and folds, which can arise from improper handling or inadequate preparation techniques, e.g., dull knifes or neglecting charging compensation [1].
Prior to sectioning, samples undergo a fixation and hardening process through freezing, resin embedding, or a combination of both via freeze substitution. This process ensures the material is sufficiently rigid to allow for high quality ultrathin sectioning [1-6]. Consequently, proper sample preparation is a vital step to ensure ultramicrotomy at the highest-quality possible [1].
Importance of Obtaining Consistent Section Quality:
The significance of consistent section quality in ultramicrotomy is essential for accurate morphological analysis and precise measurements. Variations in section thickness can distort the dimensions of cellular structures, skewing quantitative data and compromising the integrity of comparative studies [1-6]. They also influence the outcome of downstream experiments such as immunolabeling and spectroscopic analysis. Uniform sections ensure consistent exposure of target molecules or regions of interest, optimizing the efficiency and reliability of labeling procedures.
Maintaining precise section quality is fundamental for maximizing the sensitivity, specificity, and reproducibility of analytical techniques employed in conjunction with ultramicrotomy. It is even more critical in the Volume EM field, where large amount of data is used to reconstruct accurately 3D molecular structures. Data supporting this approach are often acquired from hundreds to thousands of sections collected in the form of long ribbons.
Methods
Resin (Epoxy, Araldite CY212, Agar Scientific) was polymerized according to the standard protocol. No additional biological tissue sample was embedded to focus exclusively on section quality without possible influences and bias of additional sample preparation protocols for embedding tissue. The pure epoxy-resin block was trimmed and sectioned using a UC Enuity ultramicrotome. Different section thicknesses were selected using feed setting values of 50 nm, 70 nm, and 100 nm with a sectioning speed of 1 mm/s.
Results
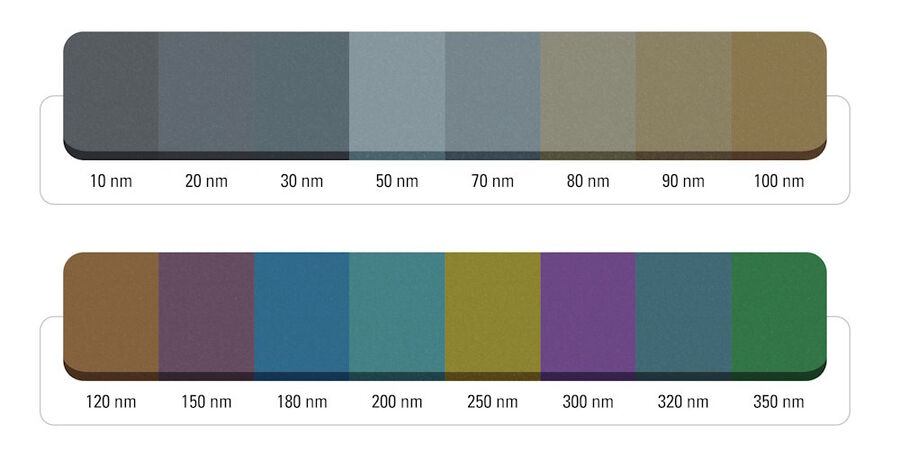
Sections cut at the nanometer scale with an ultramicrotome and floating in an illuminated water boat appear in different colors, an effect resulting from light interference. [7]. The relationship between this interference color and section thickness provides a useful visual measure [7]. A 50 nm thick section appears in a silver color, while a section with a thickness approaching 100 nm appears increasingly golden, whereas sections thicker than 100 nm exhibit an assortment of colors (Fig. 1). TEM analyses typically require sections with a thickness between 50 nm (silver) and 100 nm (gold), although in some cases, the required section thickness can deviate from this range. The color of the produced section or ribbon, visible when it floats on water, indicates its thickness [7].
Color hue vs. section thickness: A range correlation analysis
Examples of section ribbons prepared with ultramicrotomy
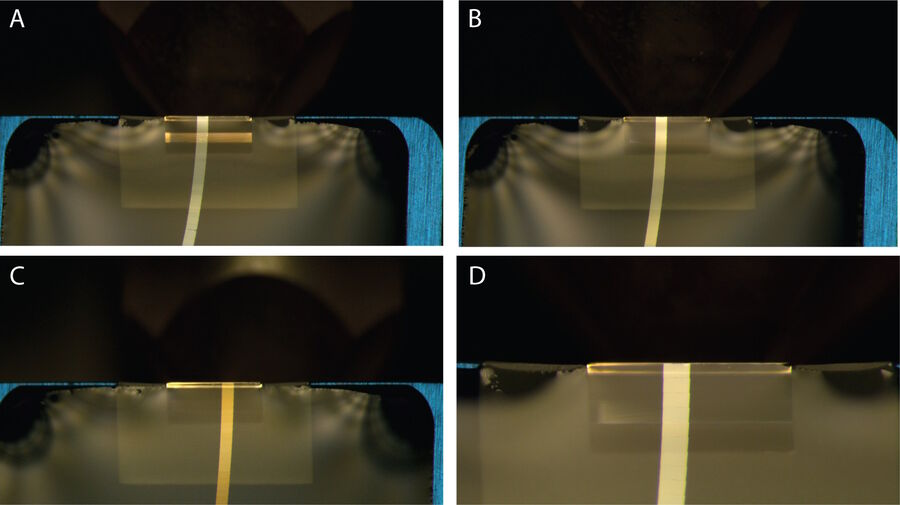
The thickness of sections can be altered by adjusting the feed step on UC Enuity [1]. However, the quality of edges is largely influenced by the speed of sectioning [1]. When the speed is too high, it can lead to unsharp edges, deteriorating the quality of sections [1]. Therefore, it is imperative to strike a balance between the feed step and sectioning speed to achieve desired sections that meet the required quality standards [1]. Typically, sectioning speed of 1 mm/s are used and recommended by the knife manufacturer (here Diatome, Switzerland)
Figure 2 demonstrates high quality sections that can be obtained with correct balance between sectioning speed and feed.
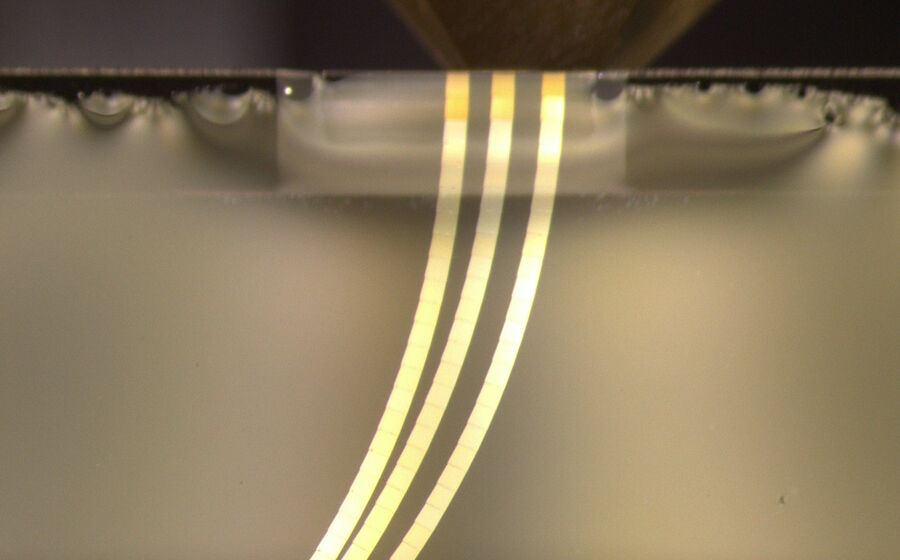
Figure 3 shows the result of a serial section workflow specifically for an Array Tomography experiment. Here UC Enuity is set to automatically prepare multiple ribbons for later collection on larger solid support such as glass slide or silicon wafer. Figure 3 shows the ribbons before collection.
The consistency of the section thickness based on the consistency in the interference color is visible across all three ribbons with 90 sections in total.
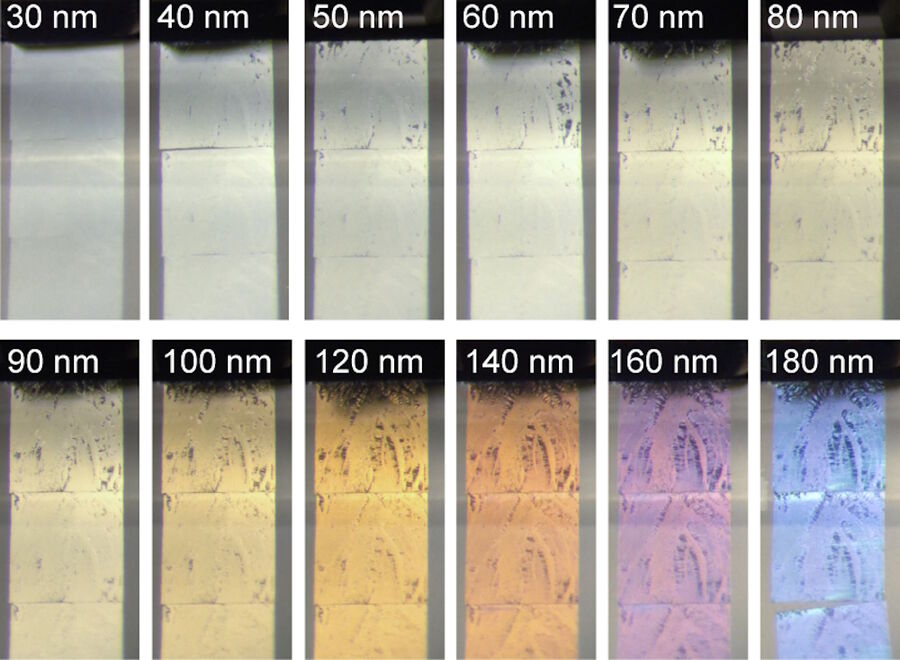
Figure 4 shows sections of tissue embedded in epoxy resin obtained with feed increasing from 30 to 180 nm. The series was not extended beyond 180 nm due to the embedded material not sectioning well at and above 200 nm. The example demonstrates the precision of UC Enuity enabling the user to seamlessly set a desired feed which results in the targeted section thickness as displayed by the interference colors (see Fig. 1)
Conclusion
Section quality is crucial for EM sample preparation. The UC Enuity ultramicrotome, leveraging over 70 years of expertise, is an essential tool for producing sharp and undistorted sections of constant thickness for EM analysis.
UC Enuity stands out for its consistent section quality and user-friendly operation, benefiting both seasoned and novice researchers. By delivering reproducible results that meet the highest quality standards, UC Enuity minimizes the variations often encountered in sectioning, facilitating straightforward comparison of experimental data. This not only helps to save time and resources but also eliminates the need for repeated sample preparation and image acquisition.