Understanding axon outgrowth after amputation
Amputation leads not only to tissue loss but also to significant changes in the nervous system near the amputation site. Axons sprouting from damaged nerves in a disorganized way can form neuromas, which can cause aberrant sensations and pain.
Advancing nerve repair through engineered muscle grafts
To try and reduce the risk of neuroma formation, a Regenerative Peripheral Nerve Interface (RPNI) can be used, where a transected nerve is implanted into a de-innervated muscle graft. This environment helps guide more natural nerve regrowth and can reduce neuropathic pain.1
Dr. Lee and his colleagues in Dr. Rylie Green’s lab at ICL are interested in whether a more sophisticated engineering solution can be designed to interface with the RPNI surgical construct.
Experimental approach
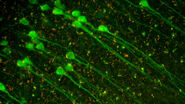
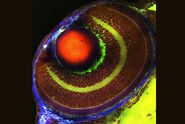
Using a rodent model, the team are able to investigate regeneration and reinnervation into free muscle grafts in a situation that would be analogous to a surgical RPNI construct.2 They have previously found that muscle tissue appears to be re-innervated from multiple sources, which they wanted to investigated further.To map nerve regeneration, the team initially used x-ray microtomography, but this lacked detail on axon formation, branching, and tissue innervation.
Investigating axon growth and tissue innervation using multimodal imaging on Mica
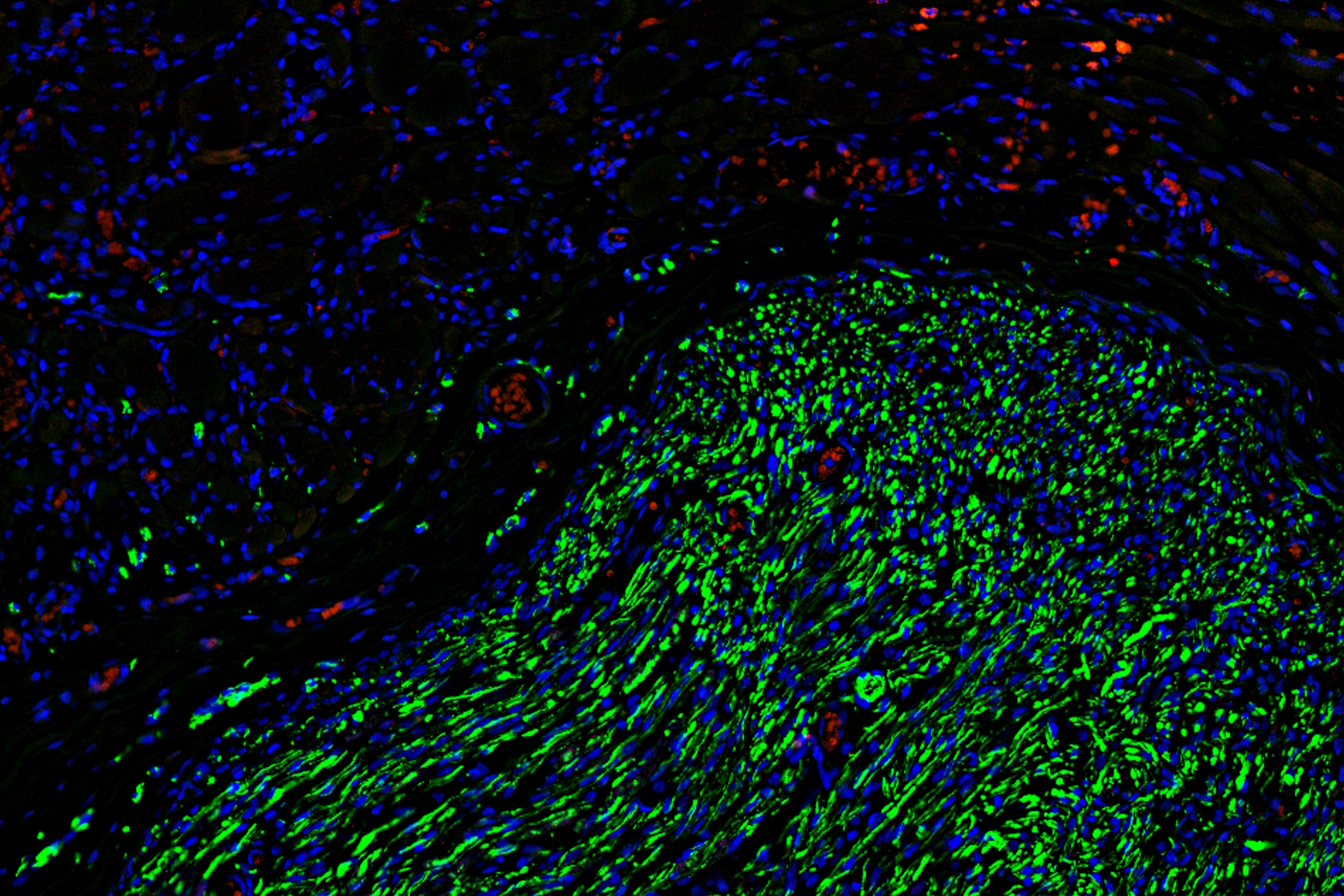
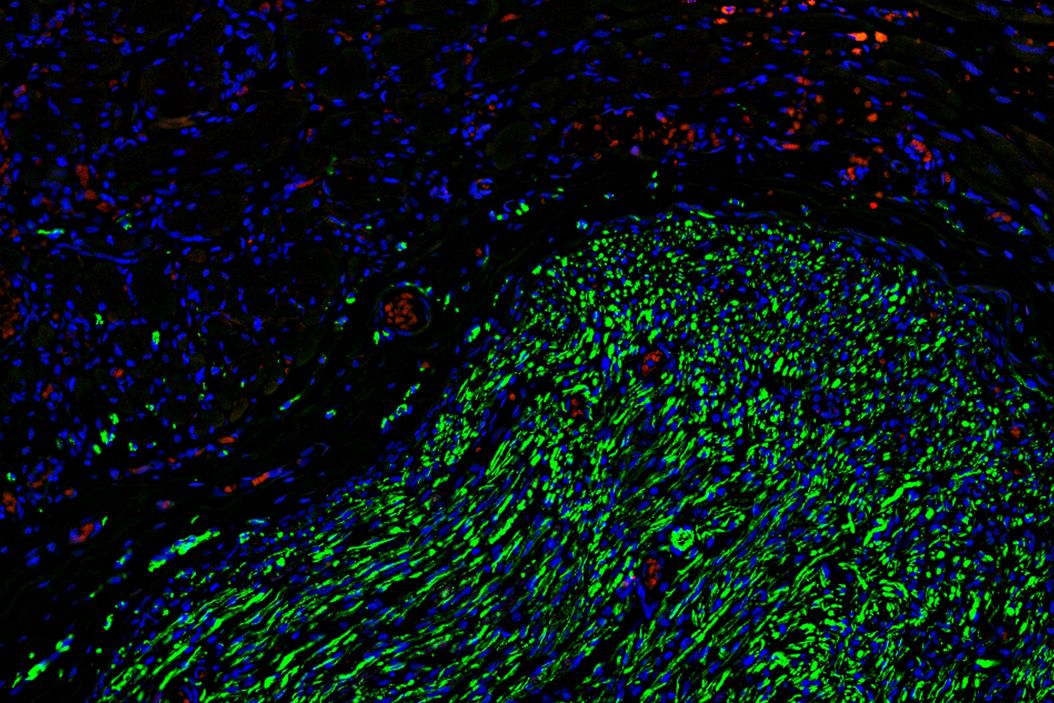
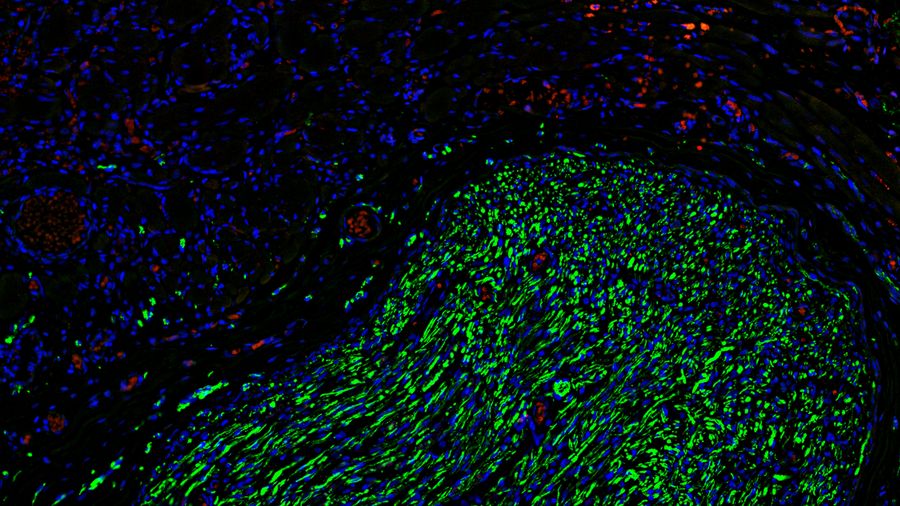
To gain deeper insights, the team turned to microscopy, using Mica to examine histological and fluorescently labeled samples. Each method offered more detailed, and complementary, insights, than the team were able to obtain before.
Using Mica, researchers were able to identify embedded nerves and investigate axonal projections deep within muscle tissues. Early trials with whole tissue 3D imaging also showed that Mica could quickly generate preliminary data on axonal trajectories, serving as a quick step in a workflow before transferring samples to a dedicated 3D imaging system.
Toward smarter neuroprosthetic interfaces
By gaining deeper insights into nerve regeneration dynamics in engineered constructs, this research could inform the design of future neuroprosthetic technology and bioelectronic devices that communicate more precisely with the nervous system. This integrated bioengineering approach could give patients a more stable bidirectional interface with greater functionality or more life-like sensations.