Reveal the structure of battery interfaces and push beyond microscale imaging into nanoscale and atomic-level analysis
If you're working on sodium metal batteries or next-generation energy storage, understanding how and why these systems degrade is essential. This session offers a detailed look at how cryogenic electron microscopy (cryo-EM) and focused ion beam (cryoFIB) techniques can reveal the true structure and chemistry of battery interfaces—without the distortions caused by traditional disassembly.
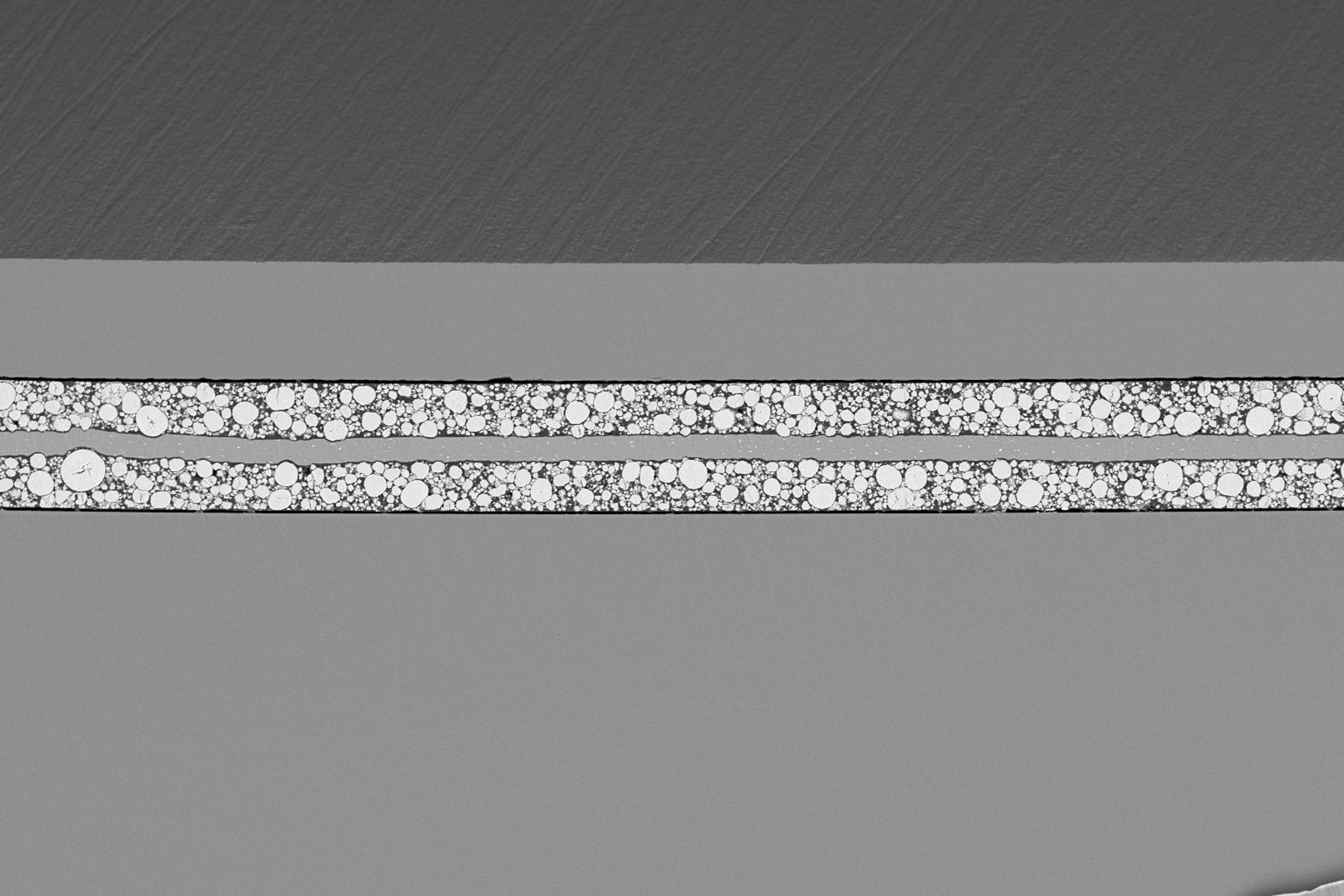
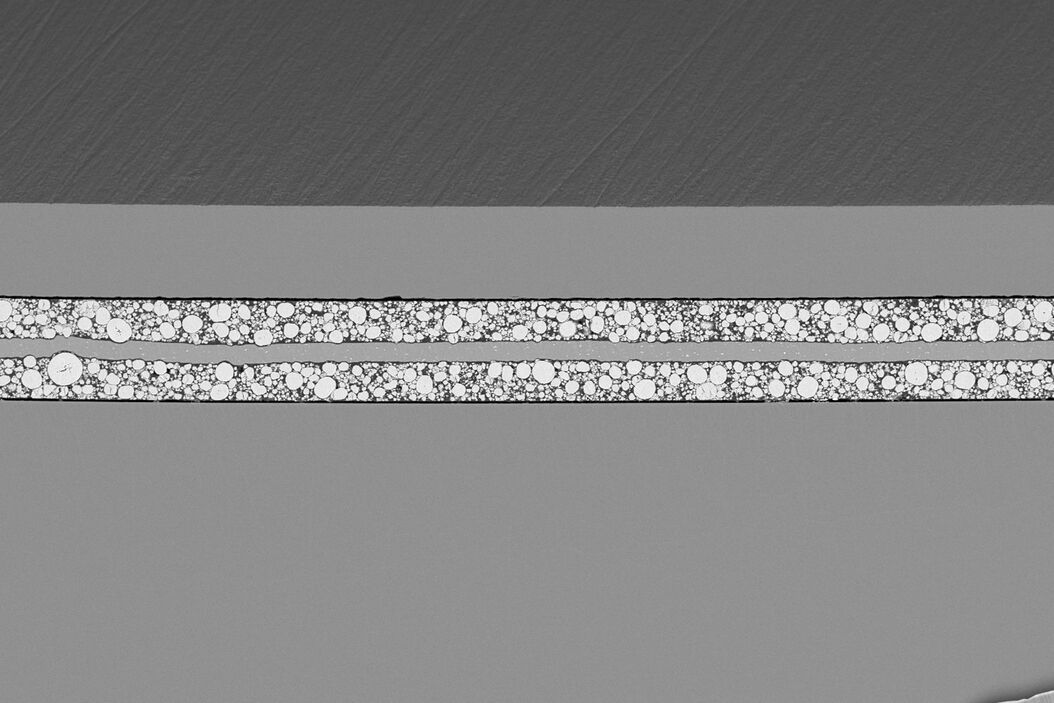
You’ll learn how sodium infiltrates the pores of polymer separators, grows laterally between layers, and eventually causes delamination. This challenges the common assumption that degradation is driven by dendritic growth and highlights the importance of separator design and pore structure.
The insights go further by comparing how different electrolyte solvents—carbonate-based vs. ether-based (diglyme)—influence degradation. You'll see how solvent choice affects SEI formation, plating uniformity, and electrolyte stability. For example, diglyme systems show more uniform behavior and less SEI buildup, while carbonate systems suffer from SEI dissolution and rapid performance loss.
You’ll also gain a clearer understanding of how degradation at the anode can influence the cathode, especially through electrolyte depletion and porosity evolution. Even in systems with better cycling stability, such as those using diglyme, oxidative breakdown at higher voltages can lead to gas formation and voids at the cathode interface.
Finally, the session explores how to push beyond microscale imaging into nanoscale and atomic-level analysis using cryo-TEM and EELS. These techniques allow you to not only identify elemental composition but also understand chemical bonding at critical interfaces. Early results show clear distinctions between bulk metallic sodium and thin oxidized surface layers—insights that are crucial for designing more stable and efficient batteries.
Whether you're developing materials, optimizing electrolytes, or studying failure modes, this content provides practical, research-backed knowledge to help you make informed decisions and accelerate your work.