Morphology
Mammalian cell culture systems can be subdivided according to several different characteristics. The most obvious one is their morphology. Due to the appearance in the microscope one can distinguish between fibroblastic or fibroblastic-like cells, epithelial-like cells, and lymphoblast-like cells.
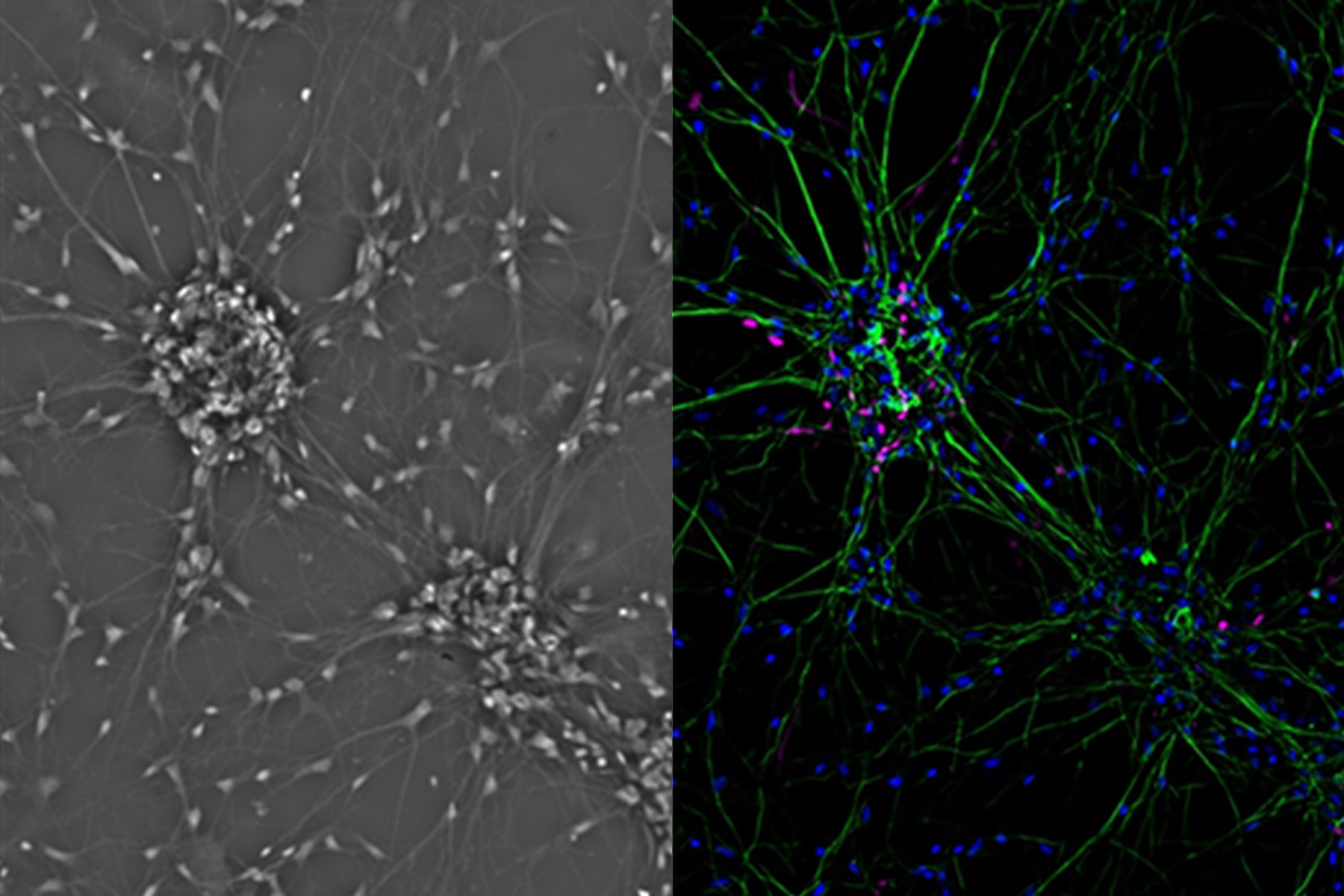
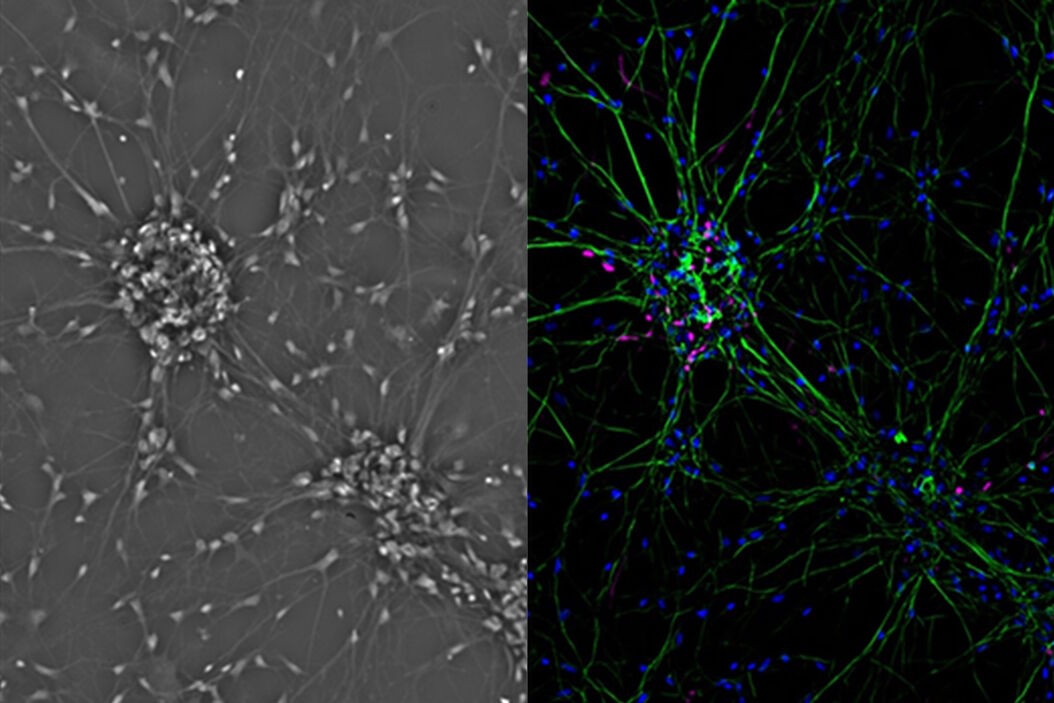
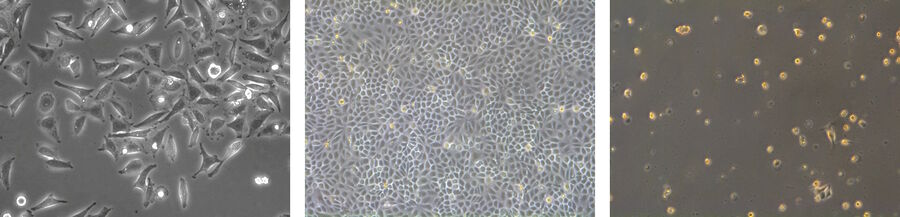
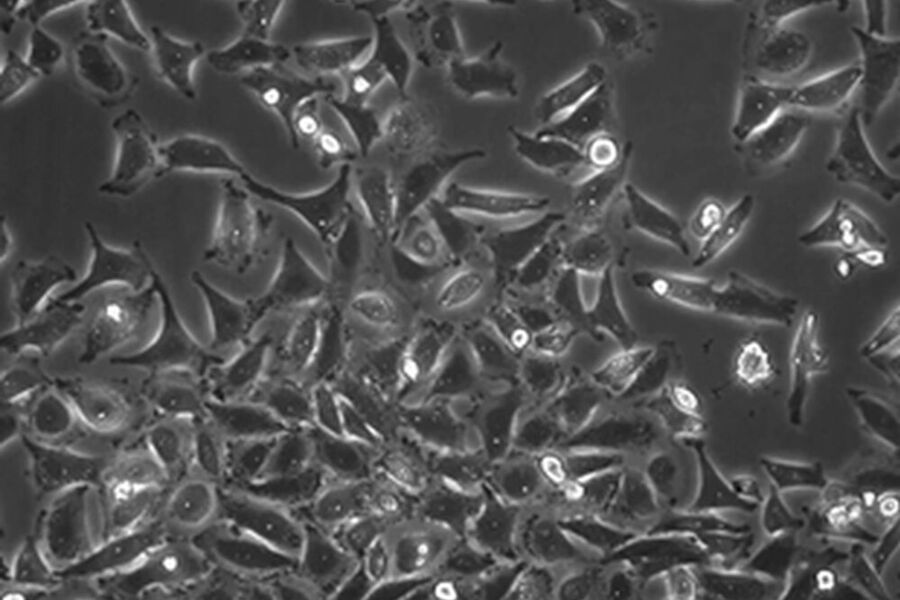
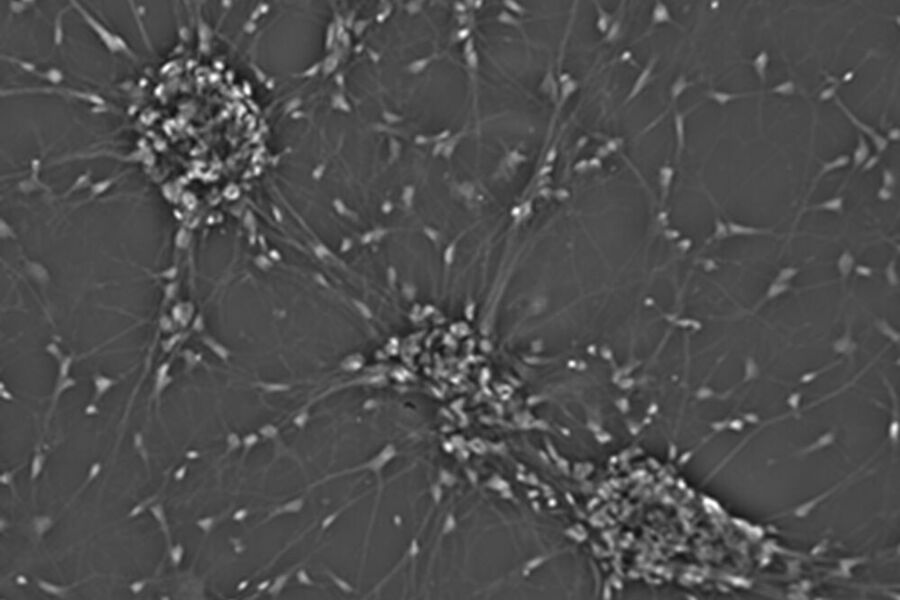
Fibroblastic or fibroblast-like cells are bipolar or multipolar and elongated in shape (Fig. 1). They grow attached to a substrate and often align into parallel assemblies. In living organisms, fibroblasts secrete the extracellular matrix and are part of the connective tissue. In addition, neuronal cells can rank among fibroblast-like cells due to their multipolar shape.
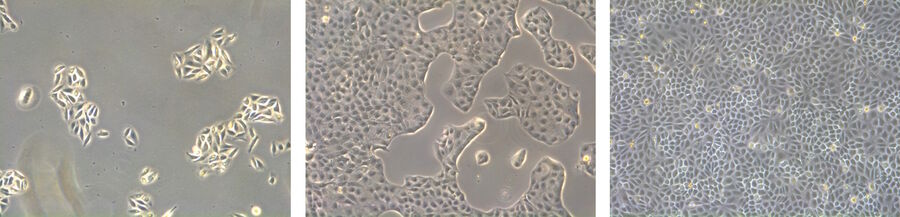
Epithelial-like cells have a polygonal shape and in general show more regular dimensions. They grow attached to a substrate in discrete patches (Fig. 1). Epithelial-like cells exhibit a plasma membrane with two sub-areas differing in structure and function. Whereas the apical membrane faces the culture medium, the basolateral membrane spreads between the individual cells and the culture vessel. Both membrane domains are separated by tight-junctions. In vivo, epithelial cells line the body structures.
Lymphoblast-like cells show a spherical outline and are typically grown in suspension (Fig. 1). In contrast to fibroblastic or epithelial-like cells they do not attach to the surface. A good example for lymphoblast-like cells are blood cells.
Cell types
Another differentiator of mammalian cell culture systems is the cell type which is used. According to their origin, cells can be subdivided into immortalized cell lines, primary cells, and stem cells (including patient specific cells).
The most common cell types in use go back to immortalized cells which were either derived from cancerous tissues or transfected with oncogenes. Due to their malignant background, they divide indefinitely. This fact makes them very easy to culture as a cell line. They are also robust and affordable.
On the other hand, one should consider that their mutated genetic background is not very close to nature any more. A very prominent example is the HeLa cell line which was derived from a cervix carcinoma. Compared to the 46 chromosomes of normal human cells, their nuclei have around 80 chromosomes. Other commonly used immortalized cell lines are HEK, A549, Jurkat, MDCK, COS, or Vero cells.
Primary cells are taken directly from living tissue. For cultivation, the original tissue fragment is dissociated enzymatically, chemically, or mechanically into single cells which can be seeded on culture flasks. Primary cells are much harder to cultivate than immortalized cell lines. Their survival rate is low and many of them don't divide. Moreover, their genetic manipulation can be challenging. Nevertheless, it might be worth the effort, because their characteristics are much closer to natural cells. In other words, scientific results which are obtained with primary cells can be translated into the in vivo world with more confidence.
Stem cells are the body’s founder cells. They can differentiate from non-specialized cells into specialized tissue cells with dedicated characteristics and functions. Thereby, they are grouped into several classes. Pluripotent stem cells are the most versatile ones and are able to differentiate into any kind of body cell. Compared to pluripotent stem cells, multipotent stem cells show a little limited differentiation range. Bipotent stem cells can only develop into two different cell types. Stem cells also divide, i.e. they can self-renew, in addition to differentiating into specialized cell types.
The source of stem cells varies. Classically they are obtained from embryos – the so called embryonic stem cells. Others can be extracted from certain tissues, e.g. the bone marrow contains blood stem cells. These kinds of cells are called “tissue-specific” or “adult” stem cells. Due to ethical concerns, gaining stem cells from living animals or humans is hard to justify. That is why the opportunity to induce already differentiated body cells to transform into stem cells gained a lot of interest. Somatic cells can be reprogrammed by transfection with only four genes (c-Myc, klf-4, oct-4, sox-2), resulting in so called <link science-lab nobel-prize-2012-in-physiology-or-medicine-for-stem-cell-research>induced pluripotent stem cells (iPSC).
Although working with stem cells is challenging, they give researchers the highest flexibility. Depending on the ingredients included in the cell medium, like certain nutrients and growth factors, stem cells can be triggered to develop into a distinct cell type, e.g. neurons.
Especially in the clinical environment, iPSCs are of great importance, as researchers are able to obtain disease specific or even patient specific cells to find a cure. This personalized medicine promises very effective therapies.
Cell culture organization
All the different cell types mentioned above can be grown in different ways. For example, a cell culture can be comprised of only a single cell type or various ones. Moreover, the cells can be organized in a 2D or 3D orientation.
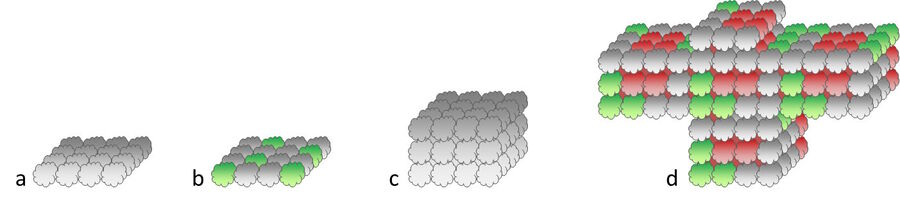
The simplest way to culture cells is in an ordinary petri dish with the cells growing on the bottom in a monolayer (Fig. 4). Such a mono culture of only a single cell type is easy and affordable. Hence, HeLa, MDCK, HEK cells, etc. which are cultured in classical containers, can be found in almost every cell biology lab. On the other hand, a 2D mono culture is also the most artificial way to maintain cells. There are two major arguments supporting this conclusion: 1) in a living organism, cells grow in three dimensions and 2) they don't grow separated from other cell types.
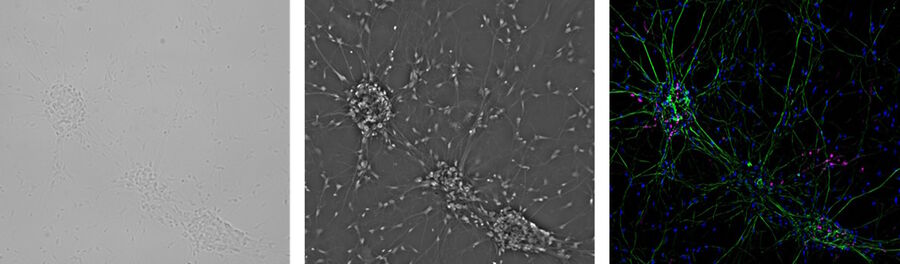
One step closer to nature would be to co-culture several cell types in two dimensions. Examples of co-culture cell line are neuronal primary cultures were glia cells grow together with neurons (Fig. 4).
To enter the 3D world, the cell biologist has to apply certain tricks. One of them is to take away the cells’ chance to adhere to the substrate. This can be achieved either by using cell culture vessels with special coatings, or the use of a gel matrix (e.g. Matrigel™), or by culturing the cells in a “hanging drop”. Expressed in a simple way, if the cells can’t stick to the culture vessel, they stick to each other. As a consequence several thousand single cells can adhere to form a so called spheroid with a diameter of 200 – 1000 µm. This kind of object has a more realistic organization including metabolic and proliferative gradients which are common in natural tissues (Fig. 4). This fact is one reason why spheroids are commonly used for drug discovery, as they mimic avascular tumors precisely.
Another way to grow cells in a more realistic 3D environment is to use scaffolds. Matrices made of metal, polymers, or ceramics can be utilized to help the cells to creep into a three dimensional form. These scaffolds can be used as a clinical implant or also for laboratory applications.
A somehow similar principle is implemented in Organs-on-a-chip which consist of a microfluidic device colonized by various cell types. The microfluidic device can have multiple chambers that can be continuously perfused. With this kind of arrangement, researchers can simulate physiology at the tissue and organ level. The additional usage of membranes and stretchable materials results in the creation of artificial structures with astonishing similarity to nature, e.g. mimicking dynamic processes like breathing or metastasis.
Another very realistic cell culture organization is based on stem cells which are cultivated in the presence of distinct growth factors inside an extracellular matrix gel. Depending on the type of growth factor (e.g. FGF, EGF etc.) and other ingredients, the stem cells develop into mini organs called organoids (Fig. 4). They resemble their “grown-up” counterparts very closely and show organotypical development and organ-specific functions. Hence brain, stomach, liver, gut, etc. organoids can be used to study diseases and organ development or run drug discovery and toxicology studies.
Growth conditions
Mammalian cell cultures have to be maintained at a temperature of 37°C. Commonly, they are cultured in special single-use plastic vessels with various shapes and sizes (Fig. 5). Among the smallest of them are 96 well plates, often used for screening applications. At the other end of the spectrum, there are cell factories exploited for large scale production of vaccines or proteins (Fig. 6). Working with cell cultures means working under sterile conditions, e.g. on a clean bench and with the use of antibiotics. Moreover, they have to be supplied with suitable media containing nutrients and growth factors. Among them are:
- glucose
- sodium pyruvate
- amino acids
- vitamins
- inorganic salts
- water
Due to its instability, the amino acid glutamine is often added in a stabilized form. Proteins, lipids, or growth factors are often supplemented in the form of Fetal Bovine Serum.
The metabolic products of the cells acidify the culture medium. Therefore, a buffer system is used to maintain a balanced pH (~7.4). The pH is commonly monitored by the indicator Phenol red. Bicarbonate (HCO3-) buffers are generally used. Their ability to bind protons (H+ ions) depends on the surrounding CO2 level. For this reason, cell culture incubators are often supplied with CO2. HEPES buffer does not depend on CO2 and is often utilized for live-cell microscopy applications where CO2 incubation is not available.
Cell culture and microscopy
When it comes to microscopy of cell cultures, there are certain basic characteristics the microscope has to fulfill. The fact that mammalian cells are cultured within liquid media means the microscope has to have an inverted configuration. In this configuration, the objective is installed beneath the culture vessel which is placed on the microscope stage and the condenser is mounted above. Only with this kind of setup can the objective come close enough to the specimen. Moreover, researchers have more free space around the sample with this kind of construction (Fig. 6).
To gain an overview on the overall status of a cell culture, a magnification of 100x – 200x is sufficient. The low intrinsic contrast of mammalian cells demands the microscope to provide specific contrast methods. Phase contrast and Modulation Contrast are the most common ones, whereas Differential Interference Contrast (DIC) is not compatible with plastic vessels normally used for cell culture (Fig. 7).
With this kind of basic equipment, researchers can judge cell number and confluency (Fig. 8). The latter describes the degree of colonization in the culture vessel (in %). Depending on the confluency, the cells have to be sub-cultured into further vessels.
In case researchers not only want to have a quick glimpse at the cells, but also document the cell culture status, then a digital microscope camera is indispensable. Moreover, mammalian cells are often transfected with genes of fluorescent proteins. To check the transfection rate, the microscope has to have fluorescence illumination capabilities, meaning an adequate light source plus the relevant filters.
References
- Liang D, Hu H, Xu T, Wang Y, Hu P, Xu Z: Generation of MERRF patient-derived induced pluripotent stem cell line iMERRF-C7. Stem Cell Res. 2016 Nov 5; 17(3):616-618
- Weiswald LB, Bellet D, Dangles-Marie V: Spherical Cancer Models in Tumor Biology. Neoplasia. 2015 Jan; Volume 17, Issue 1, Pages 1–15
- Esch EW, Bahinski A, Huh D: Organs-on-chips at the frontiers of drug discovery. Nat Rev Drug Discov. 2015 Apr;14(4):248-60.
- Xinaris C, Brizi V, Remuzzi G: Organoid Models and Applications in Biomedical Research. Nephron 2015;130:191-199
- Dekkers JF et al. Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Sci Transl Med. 2016 Jun 22;8(344):344ra84.
- Garcez PP et al. Zika virus impairs growth in human neurospheres and brain organoids. Science. 2016 May 13;352(6287):816-8