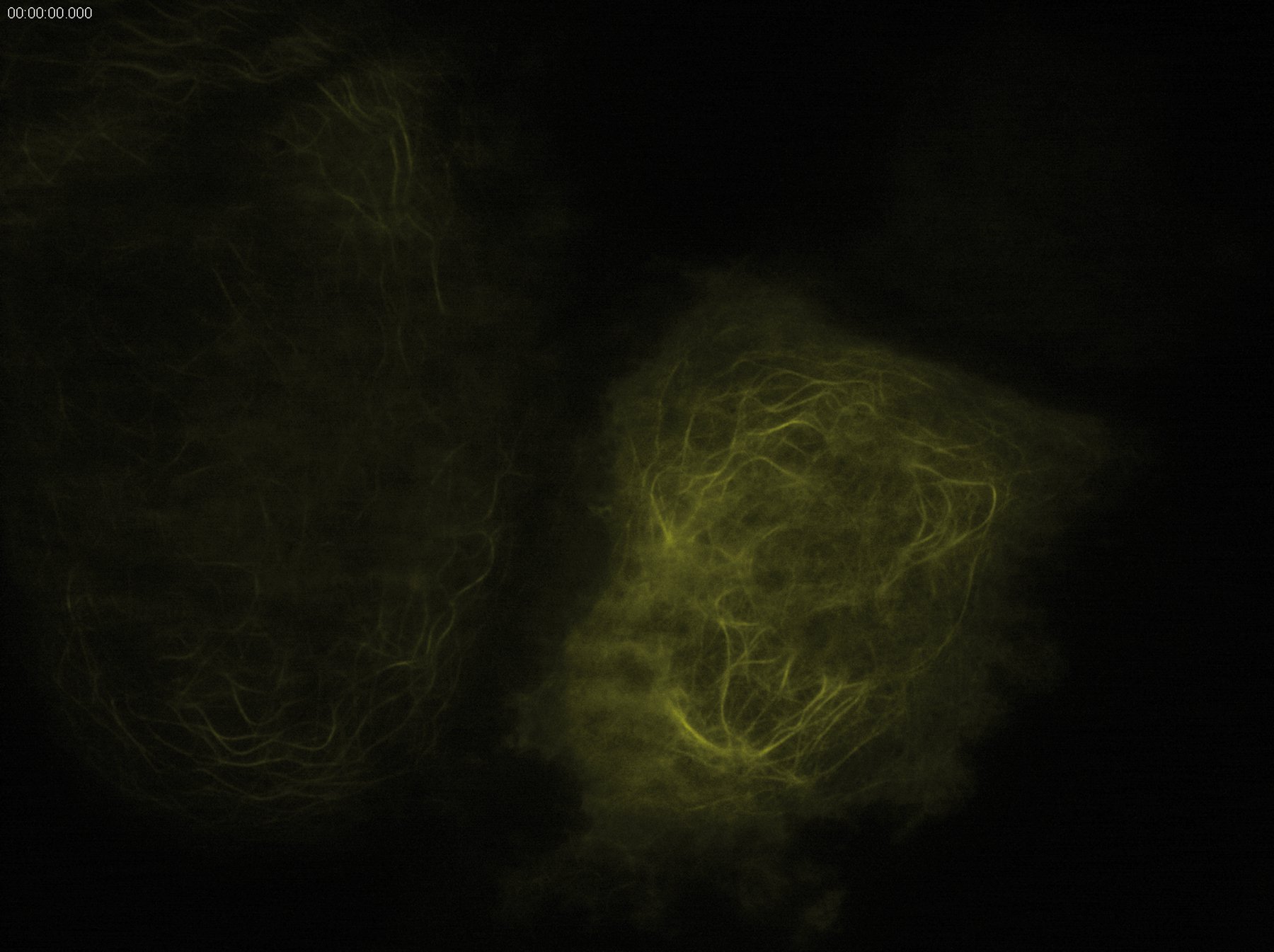
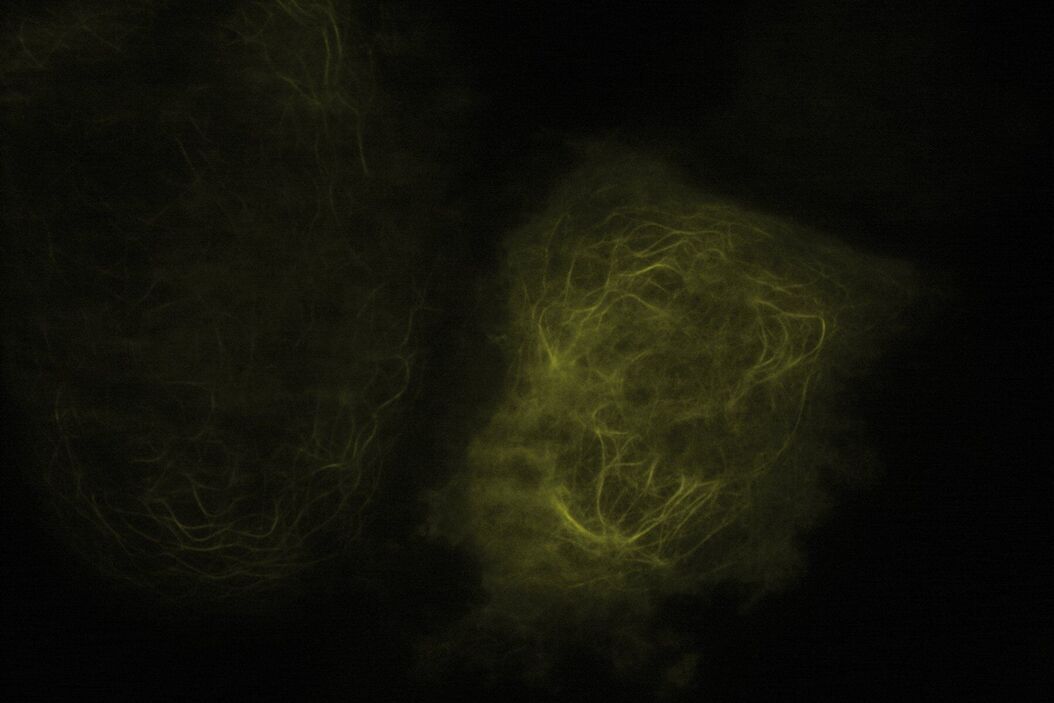
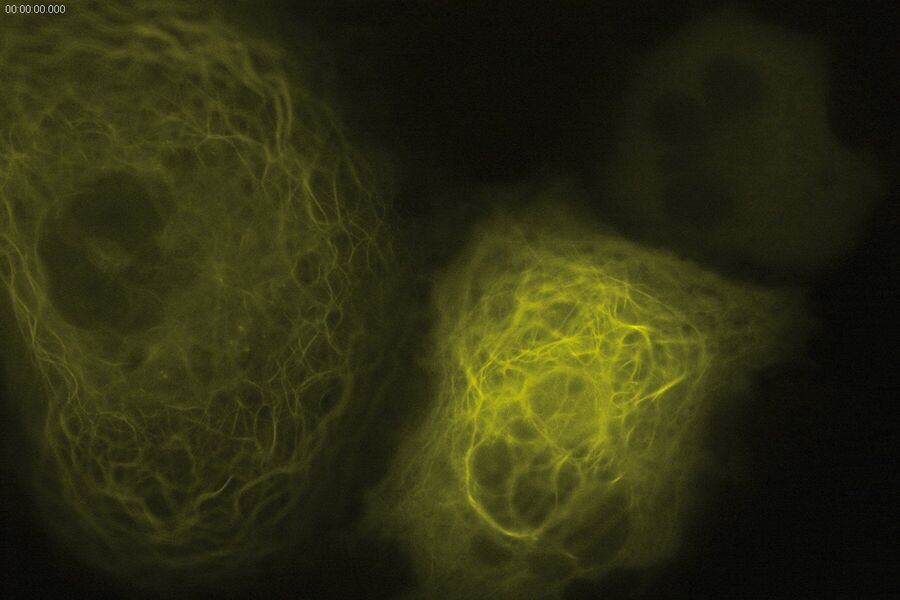
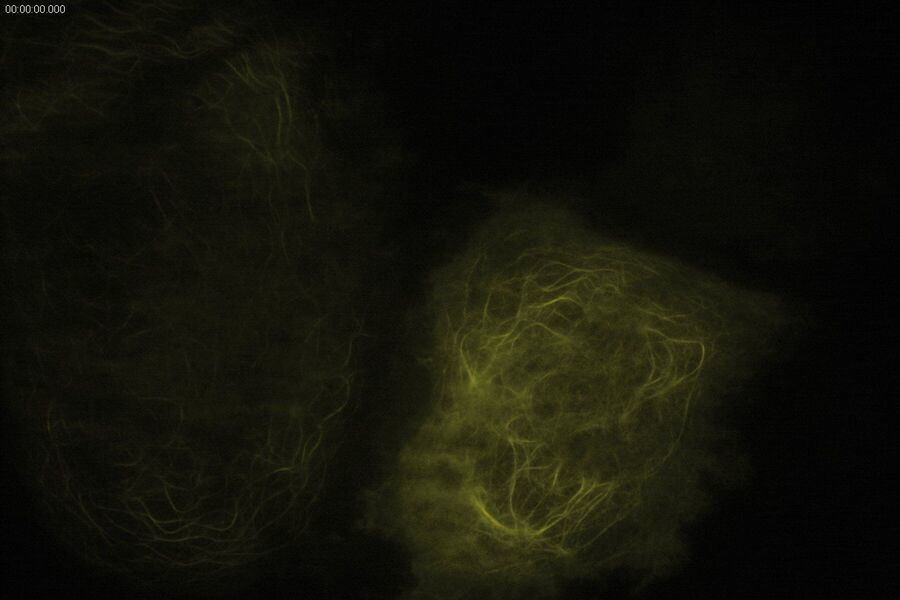
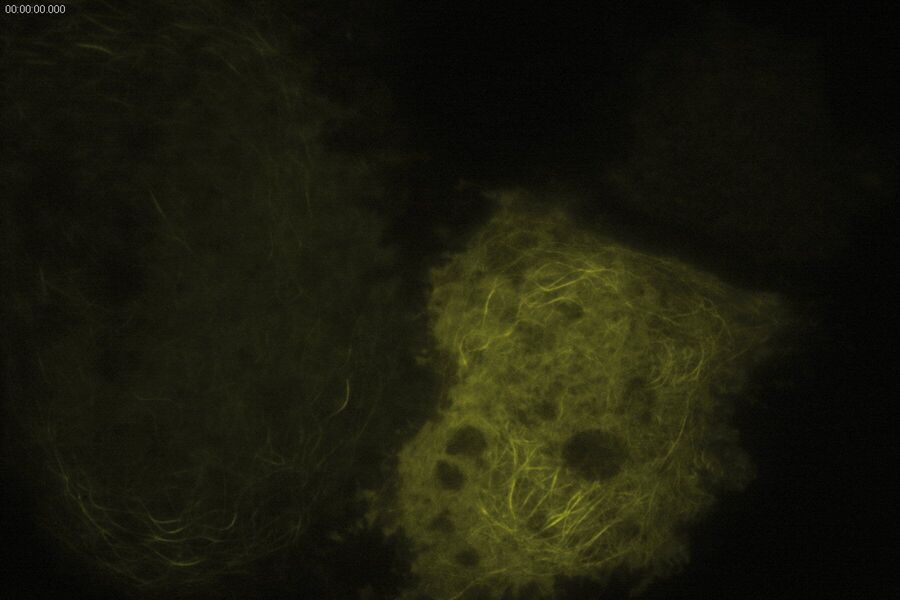
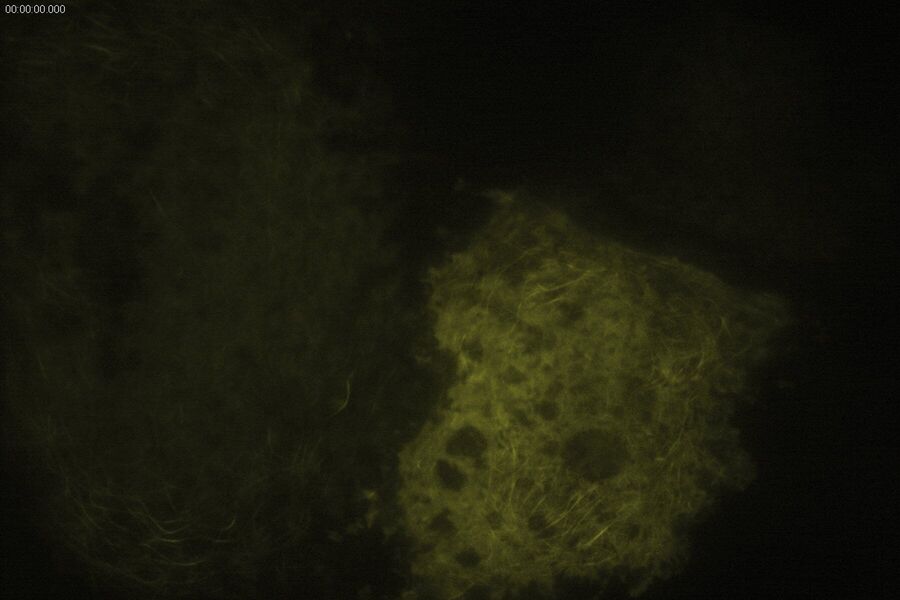
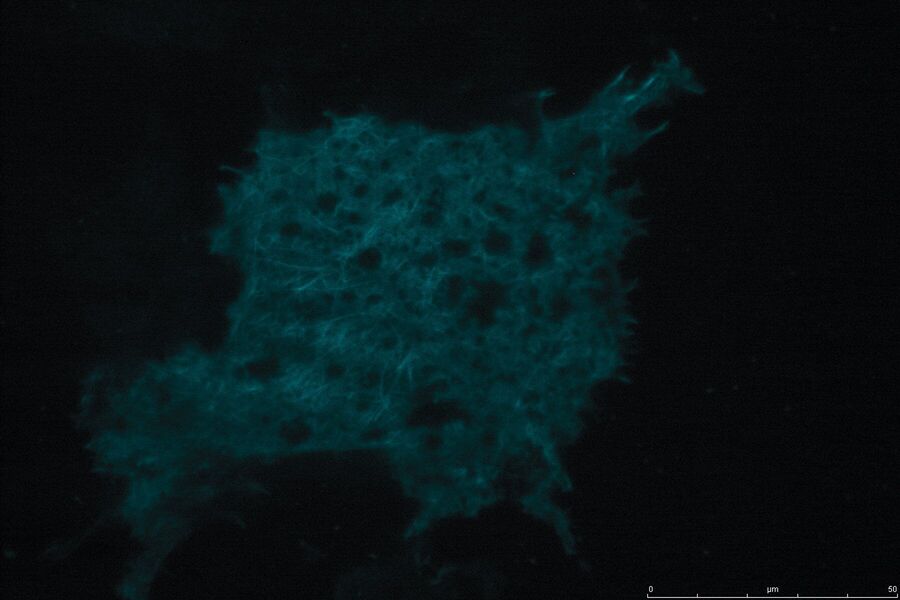
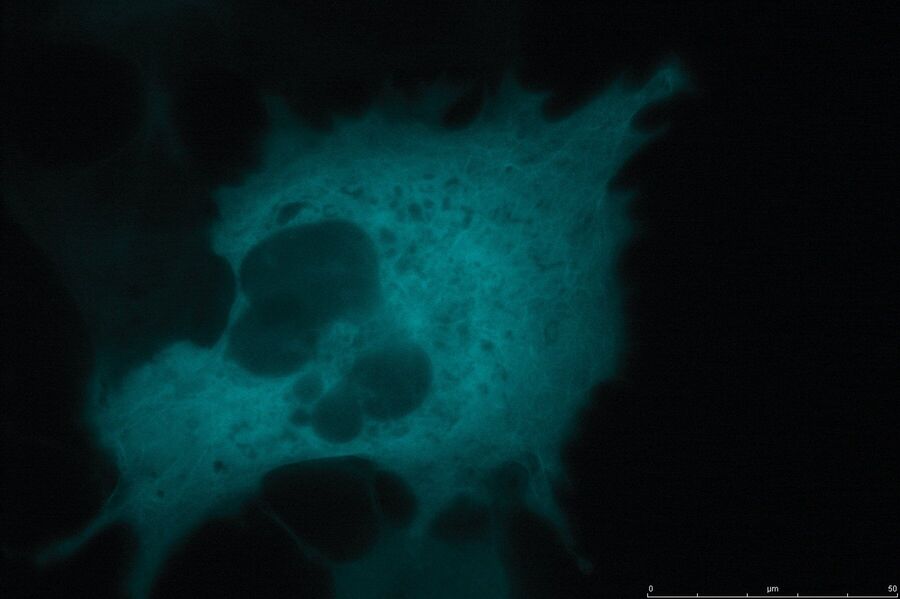
Technical advantages of TIRF microscopy
A great advantage of TIRF microscopy is its outstanding signal-to-noise ratio. Due to the low penetration depth of the evanescent field, the out-of-focus fluorescence is minimized and therefore hardly any background fluorescence occurs. As only a portion of the cell is exposed to the energy of the electromagnetic evanescent wave, the production of harmful oxygen and therefore the phototoxic stress for cells is greatly reduced as well. This dramatically improves cell viability and allows longer experiment durations. Furthermore, photobleaching at a low level only occurs in a small region of the cell. As there is a steady flux of new fluorophores from the cytosol into this region, the signal-to-noise ratio remains constant for a long period of time. Besides that, the range of fluorophores suitable for TIRF microscopy (e.g. EGFP, Fluo-4 etc.) is wide and only limited by available laser lines, so that live-cell imaging techniques like FRET, FRAP, calcium imaging etc. are easily performed. The connection of a light-sensitive camera enables high-speed image acquisition for observing fast events. Additionally, TIRF microscopy can be combined with other microscopic techniques like epi-fluorescence microscopy and brightfield contrast methods. A drawback of TIRF microscopy is that so far only adherent cultured cells can be used for experiments, as slices, for instance, are not generally close enough to the coverslip/medium interface for the evanescent wave to penetrate the specimen.
TIRF microscopy in life science research
By employing a spatially restricted evanescent field for the excitation of fluorophores, TIRF microscopy allows observation of the localization and dynamics of molecules and processes in an optical section near the plasma membrane (usually around 100 nm). This is advantageous for many applications dealing with processes in or close to the plasma membrane.
TIRF microscopy is an excellent technique for combining kinetic studies with spatial information in live samples or even in vitro. It is routinely used for investigating molecule trafficking as it occurs e.g. in cytoskeleton assembly. The rapid image acquisition and the outstanding background elimination in TIRF microscopy provide superb conditions for observing dynamic events like the recruitment of proteins to the plasma membrane. Thus, the kinetics of dynein/kinesin-mediated transport can be investigated, for example. It is also possible to track whole organelles like mitochondria using TIRF microscopy. For investigations in cell-cell interaction, special structures like focal adhesions of cells can easily be visualized with TIRF microscopy to observe e.g. the recruitment of parts of the cytoskeleton to focal adhesions.
By combining mathematical models (e.g. centroid tracking methods) with the unparalleled signal-to-noise ratio and z-resolution of TIRF microscopy, subdiffraction-limited localization of single molecules is achievable with a precision of 1 nm. This is possible as the fluorophore excitation by an evanescent wave produces low background fluorescence from out-of-focus fluorophores, resulting in a low signal-to-noise-ratio in a defined volume of the sample (e.g. the penetration depth of the evanescent wave multiplied by the area of the field of view). In a conventional lamp-based fluorescence system all fluorophores in the beam path are simultaneously excited and detected without any information about their z-position.
In other words: the three dimensional distribution of the fluorophores is displayed in only two dimensions as the different z-planes of the cell appear as one plane in the acquired image. This results in overlaying of the fluorophores in the image, which often makes the discrimination of single fluorescence spots impossible. In TIRF microscopy, however, only a comparably small number of the fluorophores within the approximately 100 nm deep evanescent wave is excited, providing an optical z-section of the sample.
The spatial proximity between fluorescence spots in TIRF images in x- and y-direction is relatively low as the light emitted from fluorophores from other z-planes is not overlaying the detected signal. If mathematical models (e.g. centroid tracking methods) are then applied to calculate the center of mass of the detected fluorescing molecule, subdiffraction-limited localization of single molecules is possible with a precision of 1 nm.
Another large field of application of TIRF microscopy is the examination of membrane-fusion processes such as vesicle trafficking. As the evanescent wave only excites fluorophores close to the plasma membrane, it is possible to monitor the formation of endocytotic vesicles as well as the fusion of secretory vesicles with the plasma membrane. For this purpose, vesicles can be labeled by tagging exocytosis cargo proteins with fluorescent proteins like GFP.
Due to the optical sectioning, it is possible to interpret changes in fluorescence intensity as motion of the fluorescence-marked vesicles into or out of the evanescent field or as a cargo release or uptake. A rather slow gain in intensity would imply a movement into the evanescent field and therefore a motion towards the plasma membrane. Upon fusion of the secretory vesicle with the plasma membrane, the fluorescent signal decreases rapidly as the cargo is released into the extracellular space. Vice versa, endocytosis can be observed by using fluorescent substrates like labeled dextran, which is taken up by cells in an endocytotic process.
Another important process that takes place at the plasma membrane is cellular signaling (e.g. signaling of G-protein-coupled receptors). Here e.g. the recruitment or movement of single molecules (e.g. G-proteins) in a signaling cascade can be observed.
All images: Courtesy of Prof. Dr. R. Jacob, University Marburg, Department of Clinical Cytobiology and Cytopathology, Marburg, Germany