Multiplexed imaging aids precision immunotherapy
Cancer immunotherapy is a promising treatment for many forms of cancer, however only a small subset of patients benefits, and relapse/recurrence frequently occurs due to various resistance mechanisms. To overcome this challenge combinatorial therapeutic strategies are needed targeting multiple steps of the cancer-immunity cycle to achieve long-term efficacy in a large population of cancer patients. It is now known that DNA damage activates the innate immune response and improves immune checkpoint blockade (ICB) efficacy. Combination immunotherapy can target several immunosuppressive elements in the tumor microenvironment and activate multiple steps of the cancer-immunity cycle. For developing an effective combination treatment strategy, including ICB with various other therapeutic modalities, we need to first understand the diverse expression pattern of various factors within tumor immune microenvironment. Multiplex immunofluorescence (mIF) is an important tool for profiling protein expression patterns in tumor tissues. This can provide a framework to understand the sensitivity to a particular combination of therapies and identify potential predictive biomarkers. Here we show spatial proteomics profiling of a diverse range of biomarkers in human colon adenocarcinoma tissue.
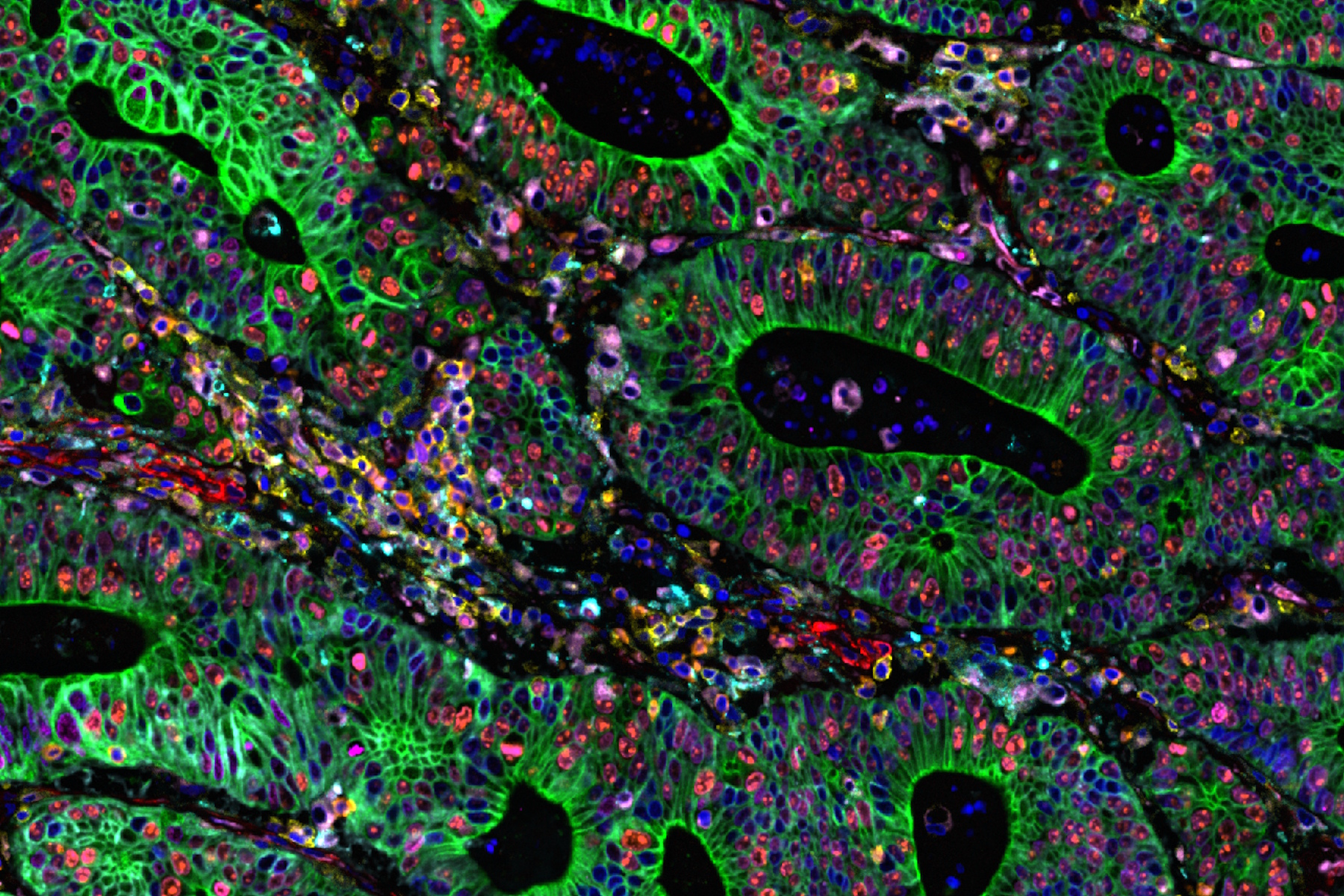
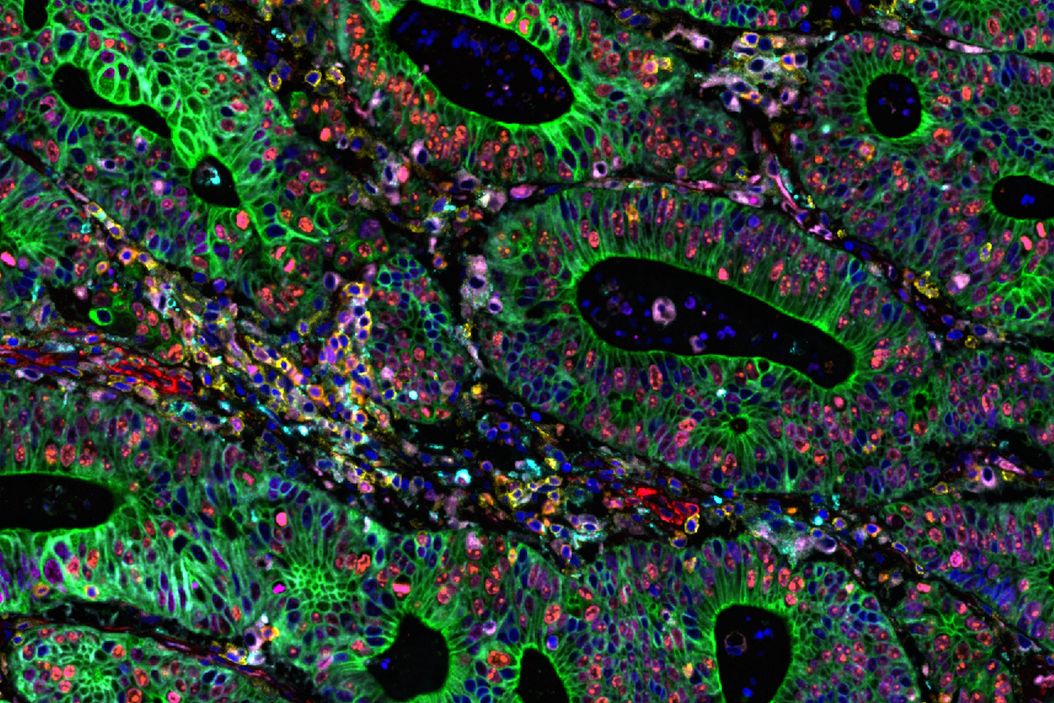
The Cell DIVE Multiplex Imaging Solution in combination with AI-based image processing software, Aivia delivers a comprehensive solution for exploring immune landscape of tumor tissue. The broad portfolio of robust IHC- validated antibodies from Cell Signaling Technology (CST) enables the detection of key proteins in the tumor microenvironment (TME). Here, we demonstrate multiplexed Cell DIVE imaging using a novel CST panel to probe human colon adenocarcinoma. The availability of cell type specific biomarkers, combined with the ability to interrogate using multiplexed tissue imaging, provides unprecedented and novel insights and spatial resolution of diverse cell populations in the TME.
AI-Powered spatial profiling of tumor immune landscape
Using Aivia software in combination with Cell DIVE multiplex imaging, researchers conducted AI-guided analysis of human colon and colon adenocarcinoma (CAC) tissues. This approach enabled high-resolution, single-cell profiling of over 30 biomarkers, offering a comprehensive view of the tumor microenvironment. Using AI-assisted segmentation and phenotyping on Aivia, the expression of all the markers within the Cell DIVE multiplexed whole tissue images was characterized (Figure 1). Advanced phenotyping, clustering, and spatial mapping revealed distinct differences in tissue architecture and cellular composition between normal and cancerous tissues. Specifically, clustering and dimensionality reduction revealed tumor-specific immune-stromal clusters and rare apoptotic/genomic instability clusters, highlighting tumor heterogeneity and immune evasion mechanisms.
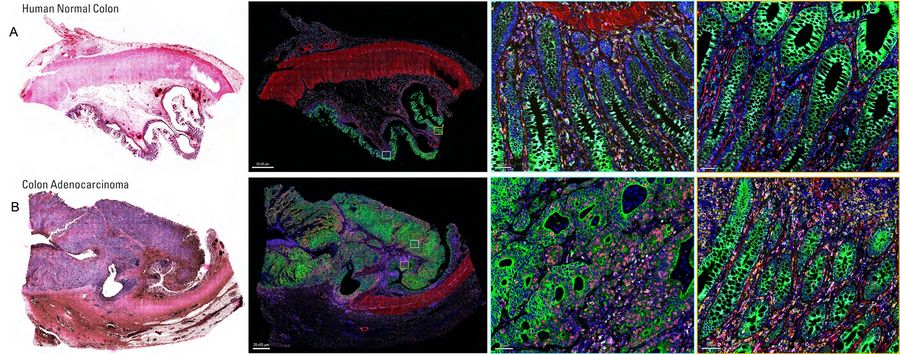
Our study shows that, high-plex spatial proteomics can help understand the interactions between cellular biomarkers and immune system. These insights can inform the development of targeted therapeutic strategies, particularly in addressing tumor aggressiveness, immune evasion, and genomic instability. Spatial mapping and AI-assisted analysis could accelerate the strategic development of combination immunotherapy regimens and optimize long-term clinical outcomes.