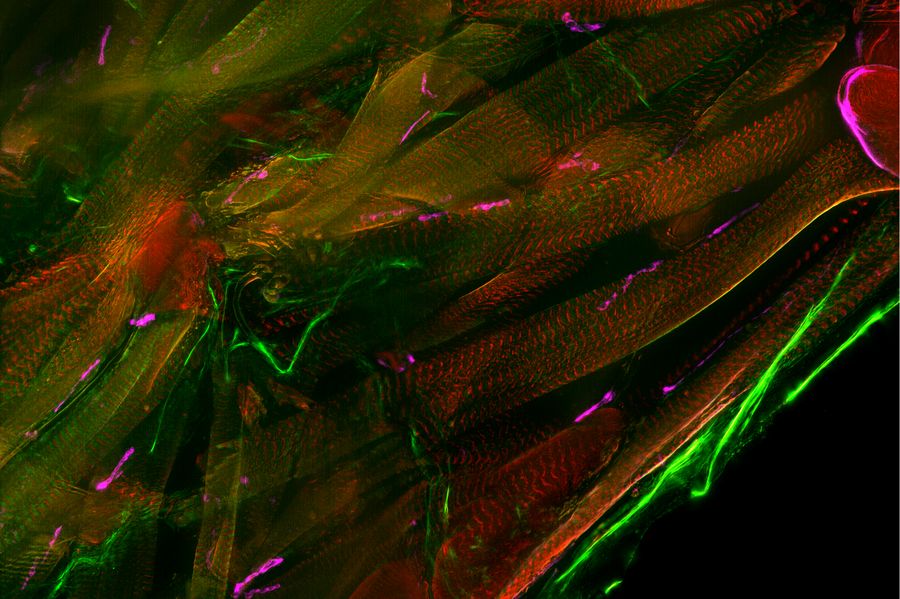
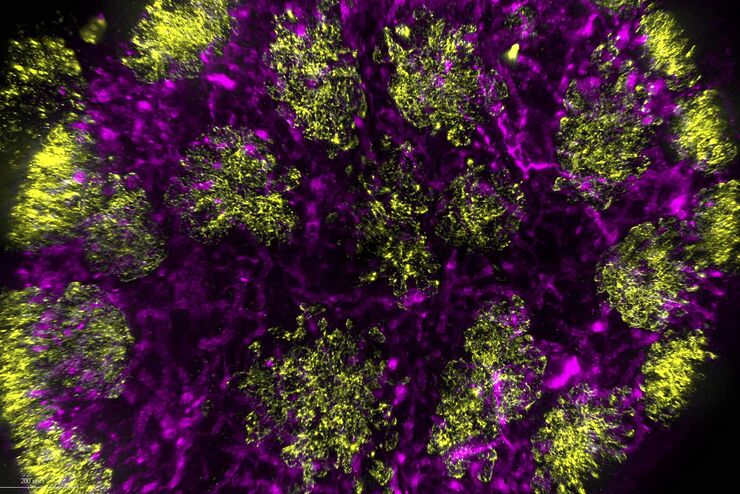
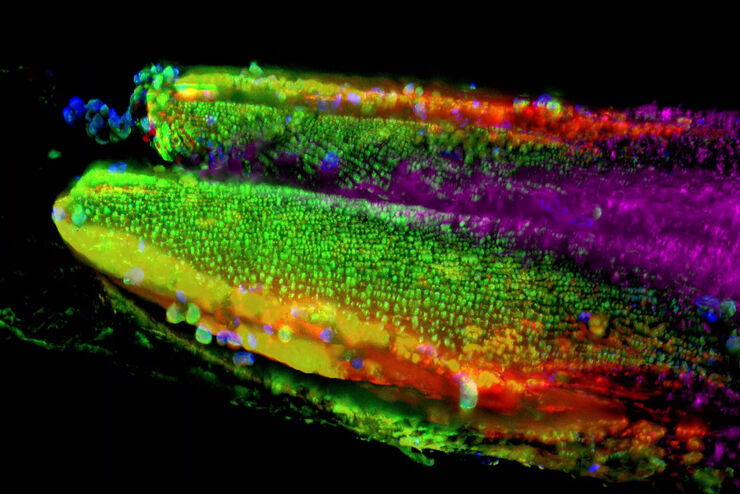
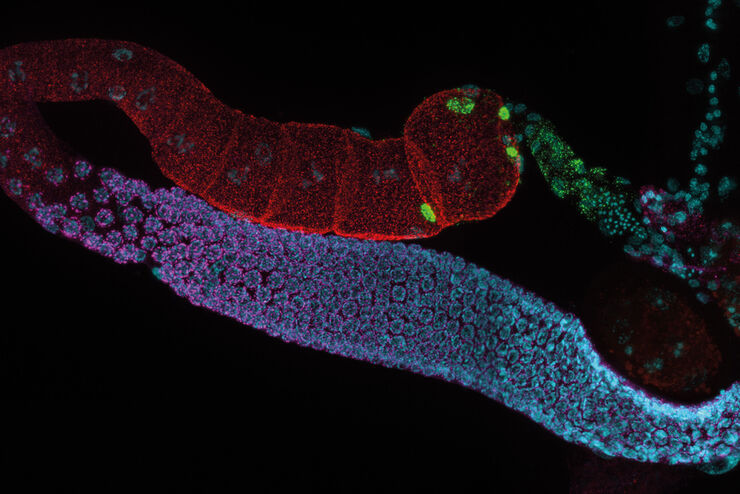
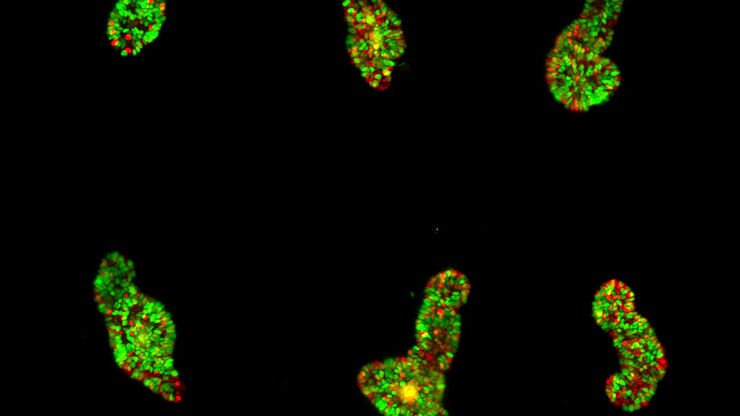
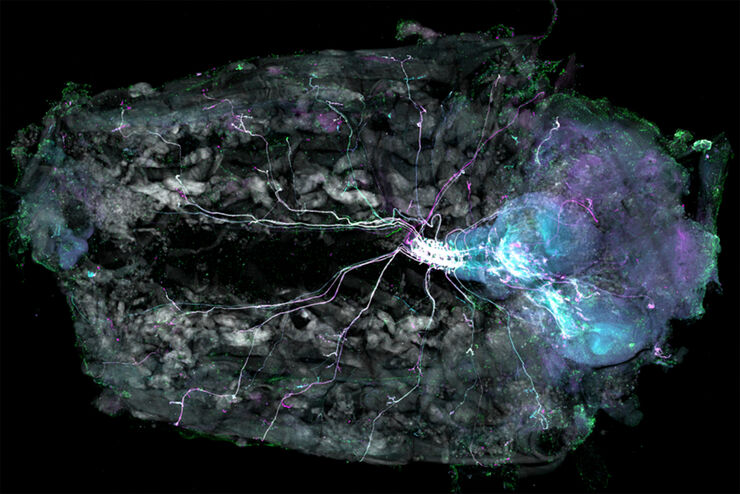
Typical Drosophila work steps using microscopes
Routine laboratory methods with D. melanogaster include:
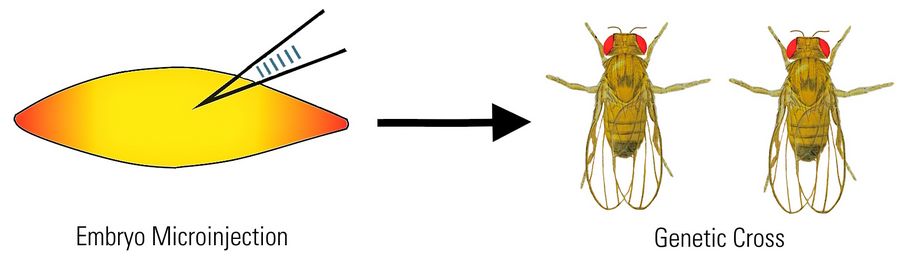
- Genetic modification (transgenesis) with microinjection of DNA into embryos
- “Fly pushing” which is the basic sorting of flies by observing anatomical characteristics
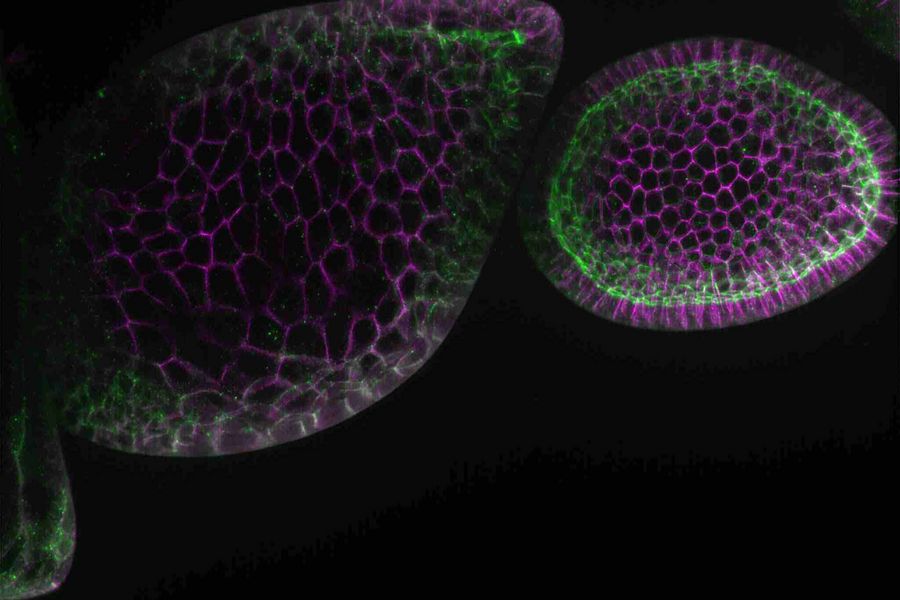
- Fluorescent screening where, for example, embryos or larvae are observed to find those showing successful transfection with fluorescent proteins
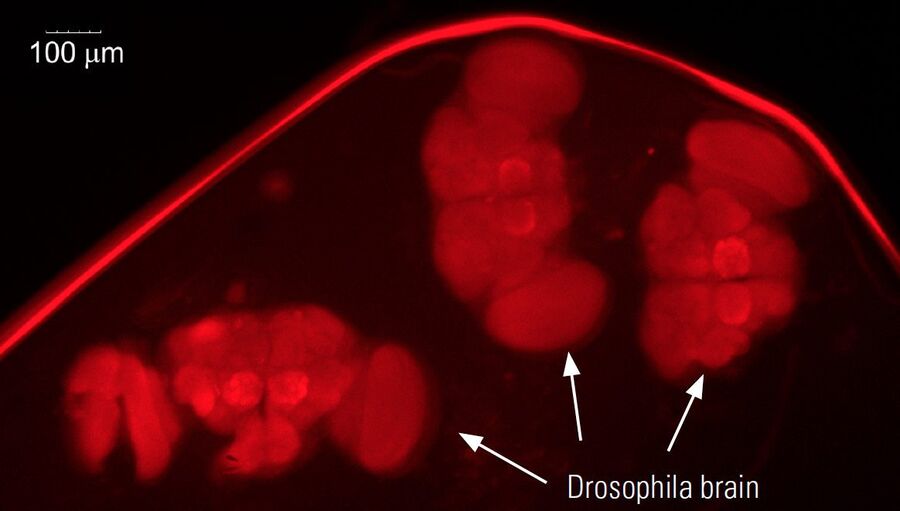
- Dissection for cases where the brain or other anatomical structures are studied
- Imaging and documentation of the screened flies (both live and fixed) or dissected anatomy during experiments.
Typically, these steps are performed with stereo, compound, or confocal microscopy.
Related Articles
Investigating Fruit Flies (Drosophila melanogaster)
Image Gallery: THUNDER Imager
Developmental Biology Image Gallery
Physiology Image Gallery
Transgenesis by microinjection
Transgenesis of Drosophila is often done by microinjection of DNA into fly embryos. To perform microinjection, a microscope is required.
There are several challenges concerning microinjection of embryos:
- Having enough space for microinjectors under the microscope objective lens
- A small field of view restricts how many embryos can be observed at the same time
- Risk of injuring the embryos
- Remaining comfortable while performing injections which require repetitive motions and can take several hours.
These challenges can be overcome by using a Leica stereo microscope, which offers users:
- A large field of view so that many embryos can be seen simultaneously
- Accessories which make the microscope more ergonomic, minimizing user fatigue and strain
- Compatibility with commercially available micromanipulators and microinjectors.
Fly pushing
The sorting of Drosophila for specific phenotypes or anatomical traits, known as fly pushing, must be done with a microscope.
The challenges associated with fly pushing are:
- Some fine details may be hard to observe
- A small field of view limits how many flies can be observed at the same
- Having both high enough magnification to see anatomical details and large enough depth of field to be able to push (sort) the flies to different groups
- A risk that the CO2 (carbon dioxide) pad, which anesthetizes the flies, is too big for the base.
However, such challenges can be overcome with a Leica stereo microscope. Users have the following benefits:
- Simultaneous higher resolution and larger depth of field due to FusionOptics
- More comfortable setup, reducing fatigue and strain, with ergonomic module
- Enough space for the CO2 pad by using a medium-sized base or swingarm stand.
Fluorescence screening
The fluorescence screening of Drosophila embryos or larvae is necessary to find those which show successful transfection with fluorescent proteins. It is performed with a fluorescence stereo microscope.
There are certain challenges associated with fluorescence screening of embryos or larvae:
- Insufficient resolution due to a low signal-to-noise ratio (SNR) can make it difficult to distinguish fine details of different phenotypes
- Often there is a compromise between resolution and depth of field
- Autofluorescence from flies and their food
- Remaining comfortable when spending long hours at the microscope.
These screening challenges can be overcome with a Leica stereo microscope. It helps users with the following:
- Reduced fluorescence background noise and increased SNR, with the TripleBeam technology
- Both high resolution and large depth of field at the same time, thanks to FusionOptics
- Reveal details clearly even deep within embryos or larvae using Computational Clearing
- Multiple contrast methods and a specially designed mirror with Leica transmitted light bases reduce autofluorescence
- Users can work comfortably with less fatigue and strain using ergonomic modules.
Dissection
Dissecting Drosophila is done when the brain or other anatomical structures must be studied. It also requires a microscope.
The challenges concerning Drosophila dissection are:
- Specimens are small and fragile, so it can be difficult to see internal structures
- During brain dissection, contact with air should be avoided
- Achieving both speed and precision can be difficult
- Reflections when dissecting flies in a solution.
It is possible to overcome the dissection challenges with a Leica stereo microscope. Users can take advantage of:
- Easy orienting of fly specimens in 3D with stereo microscopy
- Working in comfort with less fatigue and strain thanks to ergonomic modules
- Reflections from a solution surface can be greatly reduced with polarizers and various illumination options.
Related Articles
Selecting the Right Dissecting Microscope
Key Factors to Consider When Selecting a Stereo Microscope
Imaging and documentation
After screening and possibly dissection of Drosophila, imaging and documentation of experiments often follow. For this step, a microscope with a camera and software is needed.
The main challenges concerning imaging and documentation of Drosophila experiments are:
- Avoiding damage to the fly specimens from light and heat exposure, especially during live imaging
- Movement of the specimen out of the field of view
- Out-of-focus blur when imaging thick structures or those deeper in specimens due to light scattering
- Obtaining an in-focus 2D image of a 3D specimen.
These challenges can be overcome with a Leica microscope. Here are the advantages:
- Record fast events with a microscope camera having high frame rates and sensitivity
- See details deep within fly specimens rapidly thanks to Computational Clearing
- More gentle exposure of the fly specimen to light with widefield microscopy
- Acquire an in-focus image of a whole Z-stack with extended depth of field (EDOF) imaging
- Obtain higher resolution images of fly specimens with light sheet and confocal microscopy.
Related Articles
Live-Cell Fluorescence Lifetime Multiplexing Using Organic Fluorophores
How to Study Gene Regulatory Networks in Embryonic Development
Dual-View LightSheet Microscope for Large Multicellular Systems
How Microscopy Helps the Study of Mechanoceptive and Synaptic Pathways
A Guide to Spatial Biology
Understanding Motor Sequence Generation Across Spatiotemporal Scales
FAQs
The life cycle of Drosophila is about 3 weeks. Embryonic development, after fertilization and zygote formation, takes place in an egg. Larva eventually come from the eggs and grow to become pupa. Then, the pupa develops into adults.
The Drosophila is extensively used as a model organism for the fields of genetics, cell biology, biochemistry, developmental biology, and neuroscience. Before its genome was sequenced in the year 2000, it had been used since the early 1900s to advance our understanding of genetics.
About 60 percent of all human genes and 75 percent of human disease-associated genes have equivalents, orthologs, in Drosophila. Also, there is the simplicity of the fruit fly's genome with only 4 pairs of chromosomes (there are 23 in humans). The male and female flies are easy to tell apart compared to other model organisms.
Scientists use the following methods to genetically modify the fruit fly known as Drosophila: targeted mutagenesis by homologous recombination, CRISPR/Cas9 (clustered regularly interspaced short palindromic repeats / CRISPR-associated protein 9), zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), prime editing, and site-specific transformation via PhiC31 integrase. To express fluorescent proteins in Drosophila, the UAS/GAL4 system, split fluorescent protein systems, flybow system, or enhancer trap screens are often used.
It is convenient and cost-effective to use the Drosophila for disease research, because large-scale genetic screens of mutant flies can be done relatively quickly.
About 60 percent of human DNA is identical with the DNA of Drosophila melanogaster. The common genes between humans and the fly concern processes involved with growth and development. About 75 percent of genes that cause disease in humans are also found in Drosophila, so they are good model organisms for the study of disease.
The fruit fly known as Drosophila melanogaster is a model organism used for genetic studies and other research concerning biology. It shares with humans 75% of genes causing disease. With a malleable DNA code and the ability to breed quickly, Drosophila helps scientists understand disease more efficiently than human models.
Drosophila melanogaster is often called a fruit fly or, less often, a vinegar fly. D. melanogaster is attracted to rotting fruit, food, and beverages. They can be found in gardens, orchards, kitchens, garbage containers, etc.