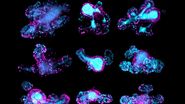
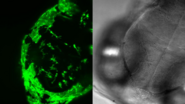
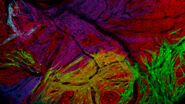
Screening of transgenic zebrafish embryos
Transgenic zebrafish embryos are common model organisms used in developmental biology [1]. These samples are often engineered for in vivo volumetric imaging using systems requiring specific sample preparation including light-sheet microscopy [2]. Therefore, a rapid and accurate screening technique is required to determine if appropriate protein expression is present for zebrafish downstream imaging with light sheet microscopy.
Zebrafish screening is often performed using stereomicroscopes with fluorescence imaging capabilities. However, a limitation of stereomicroscopy is reduced resolution and sensitivity, which becomes particularly important in samples where expression is low. Conventional widefield microscopy is a high-speed imaging technique, but also suffers from out-of-focus blur, particularly for thicker samples including zebrafish embryos. This out-of-focus blur reduces contrast which is crucial to visualizing the expression in zebrafish.
The DM6 B microscope equipped with THUNDER is a user-friendly system that facilitates high-speed screening through:
- High-speed triggering with the New SynapseTM electronics;
- Gentle and targeted illumination using the 8-line LED source;
- Detection of low expression proteins thanks to the high sensitivity K8 camera;
- Fast overview scans to locate optimal transgenic zebrafish via the LAS X Navigator spiral scan;
- Improved contrast and clearly visualized fluorescent proteins due to THUNDER Live Instant Computational Clearing.
Materials and methods
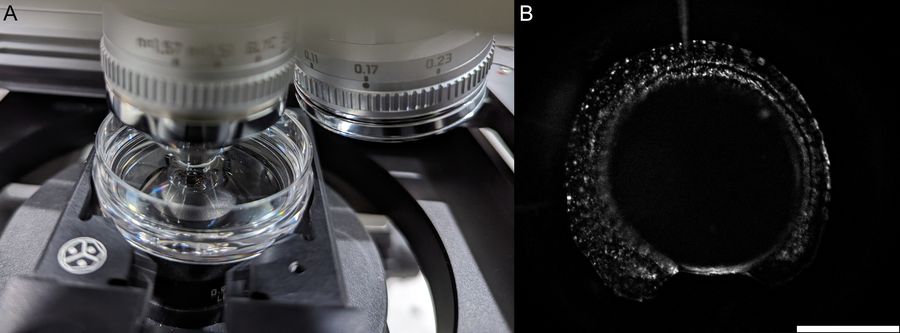
- In this workflow, transgenic zebrafish are transferred into a 35 mm petri dish. However, other mounting platforms also exist that are compatible with the THUNDER Imager Tissue.
- Low magnification objective is used to locate zebrafish embryos in a petri dish (Figure 2).
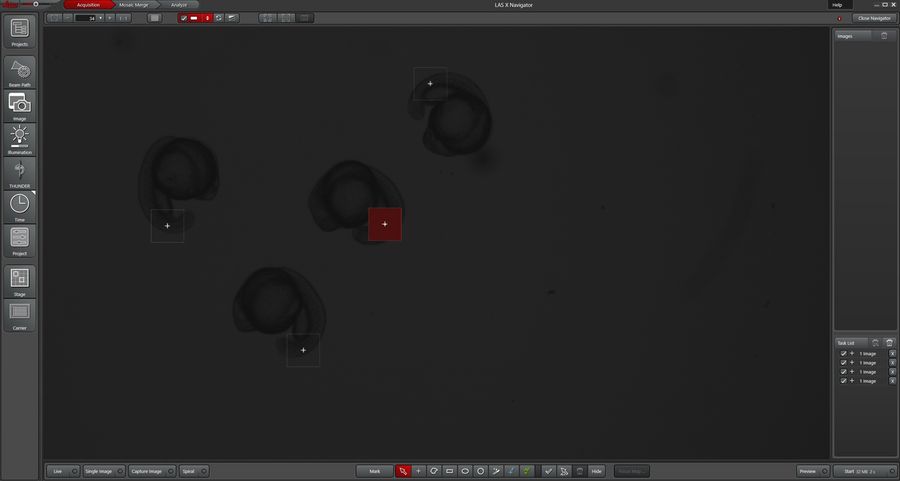
- LAS-X Navigator is used to mark regions of interest for Zebrafish (Figure 3).
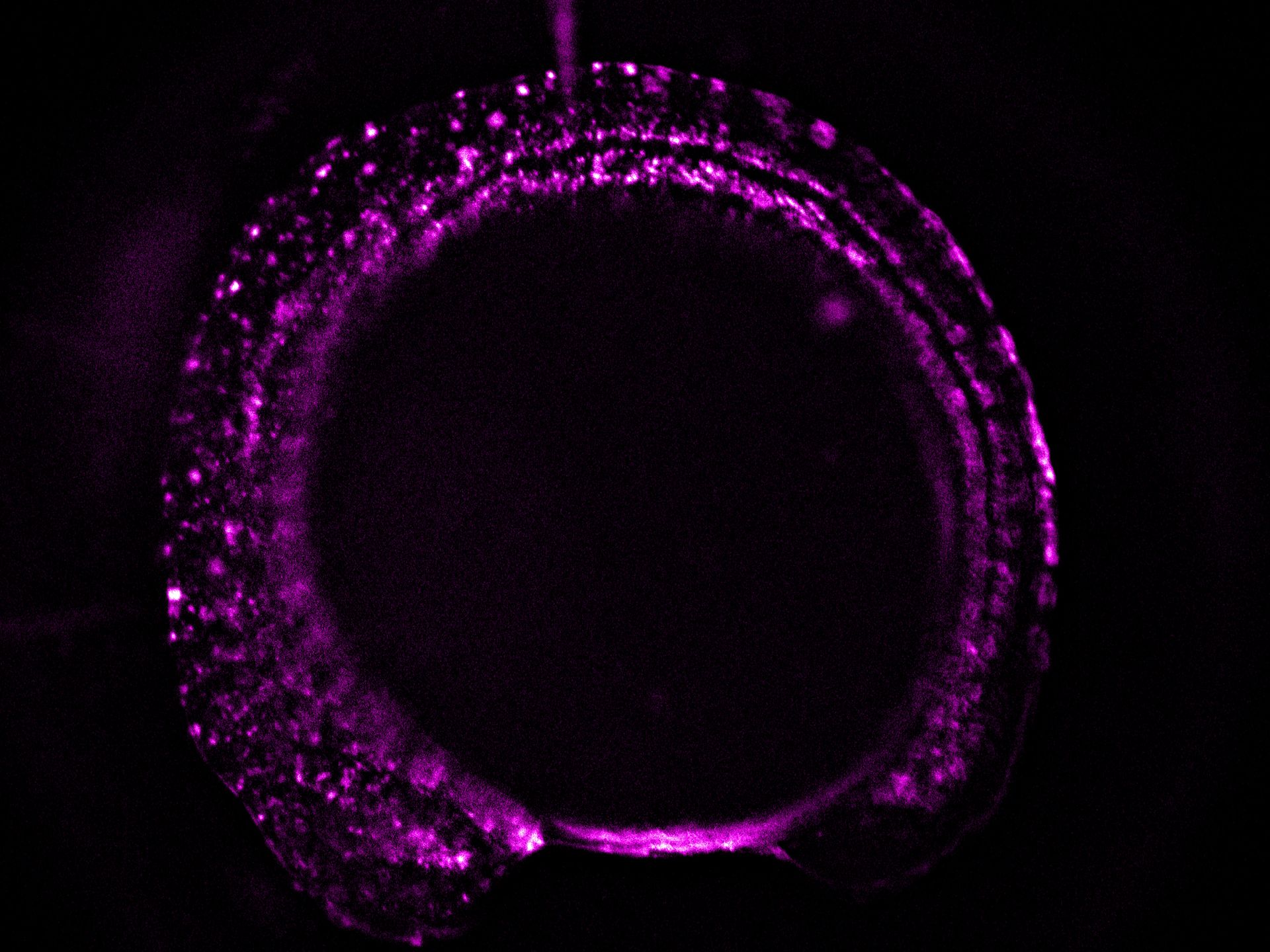
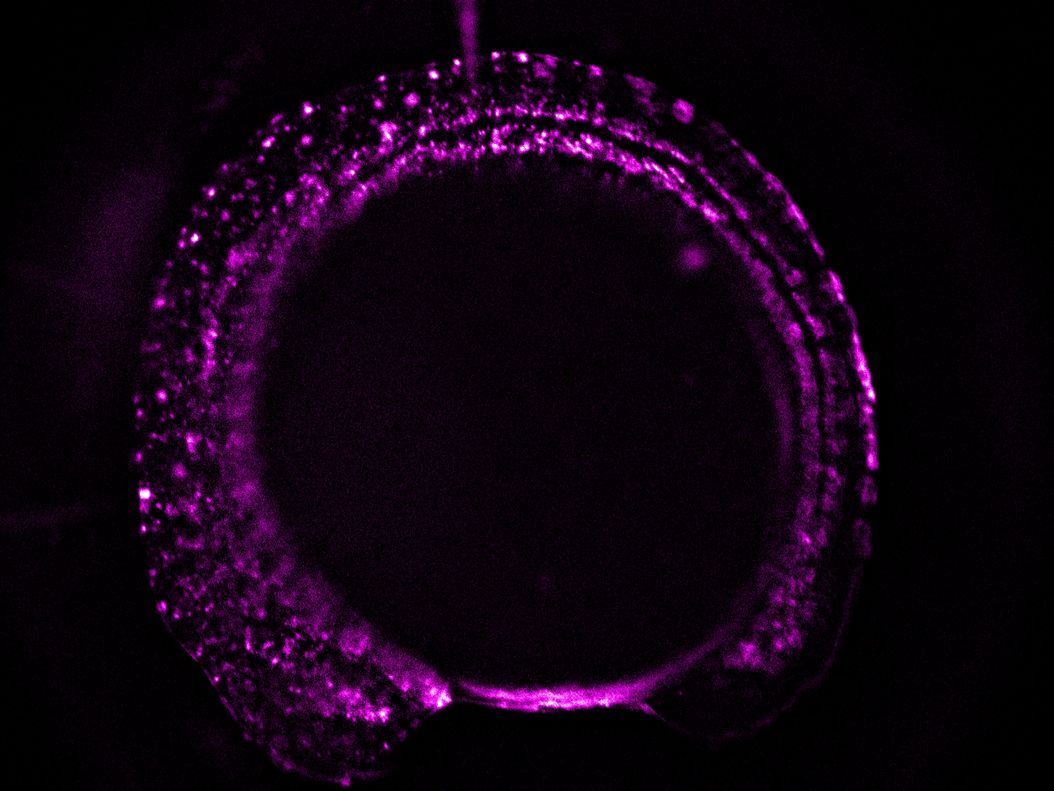
- A higher resolution objective is used to determine if expression is sufficient for downstream imaging. Such objectives include HC FLUOTAR L 16x/0,60 IMM CORR VISIR (506533), HC APO L 63x/0,90 W U-V-I (506148), and HC FLUOTAR L 25x/0,95 W VISIR (506374). The combination of low LED light dosage, 95% peak QE of the K8 Scientific CMOS camera, and real time triggering ensures endogenous level protein signals are preserved during screening (Figure 4).
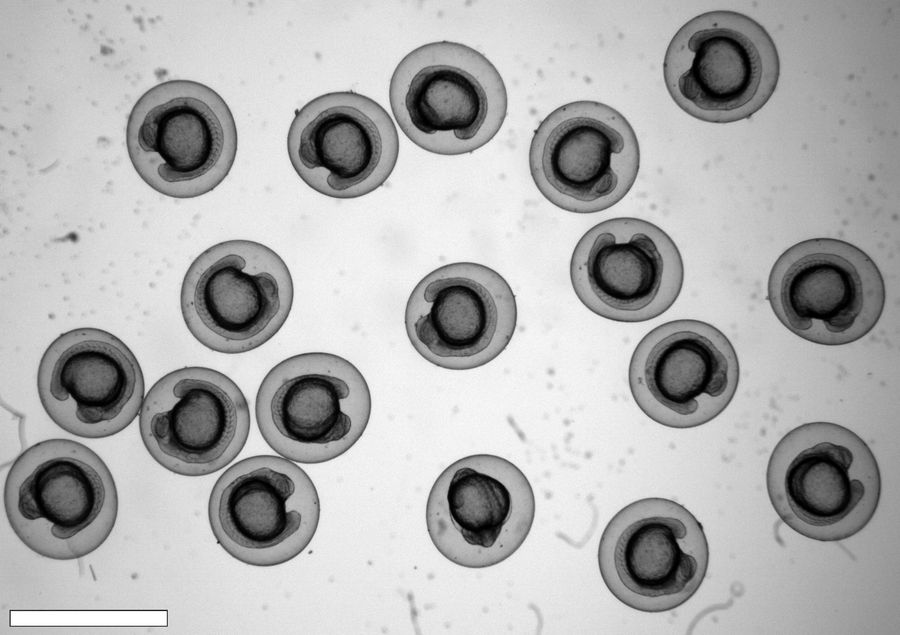
Conclusion
Zebrafish embryos with appropriate transgenesis are selected for downstream experiments including light-sheet imaging. The high-speed imaging capabilities of the DM6 B equipped with THUNDER technology and combined with enhanced contrast and sensitivity for imaging low protein expression, help ensure the accurate and efficient screening results. This study demonstrates that the DM6 B and THUNDER technology can help improve the understanding of phenomena in developmental biology and other related fields.