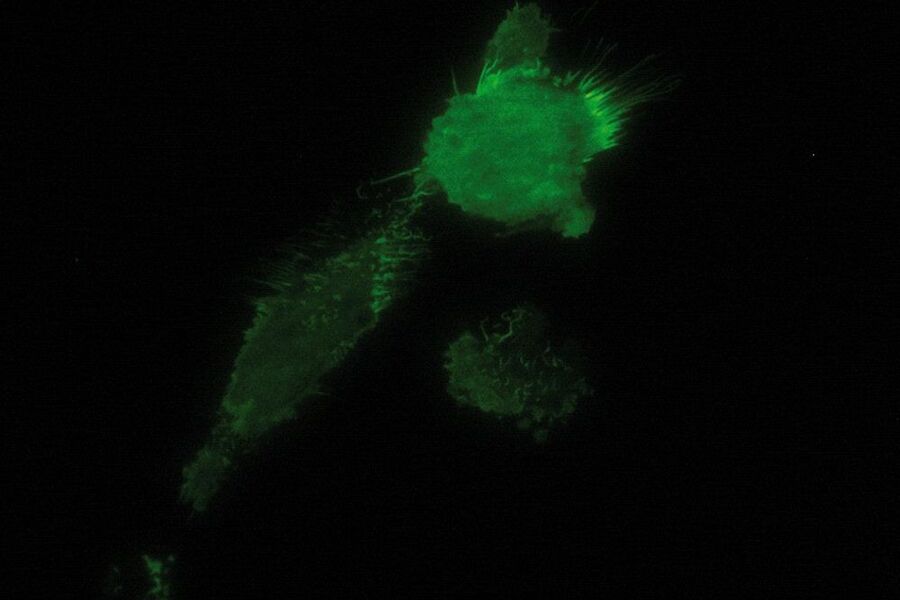
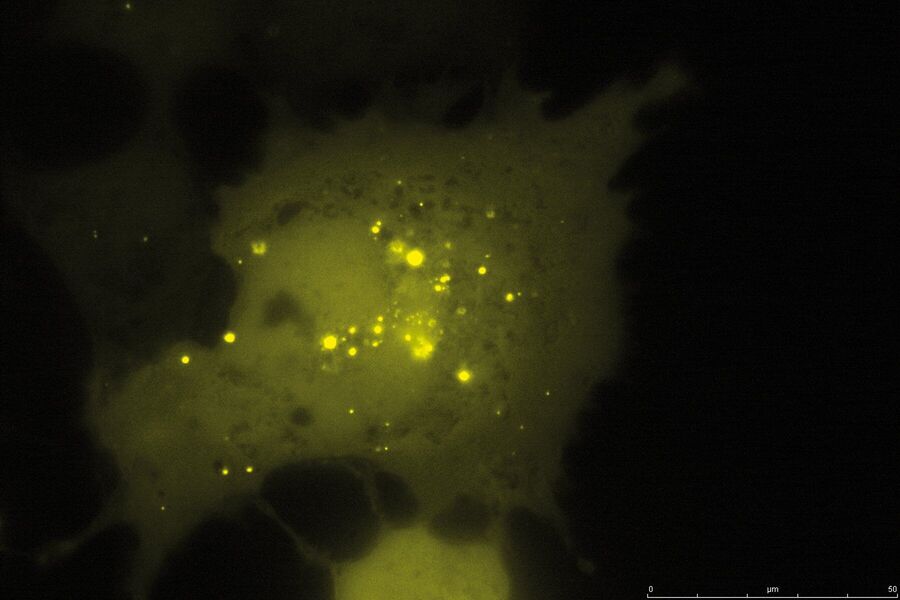
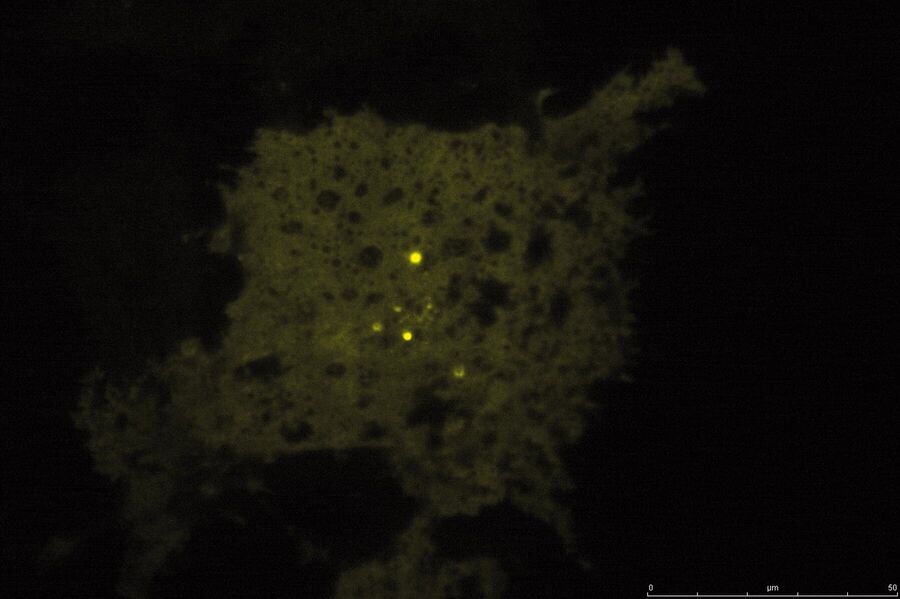
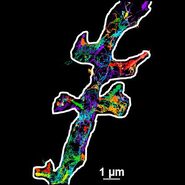
TIRF Microscopy
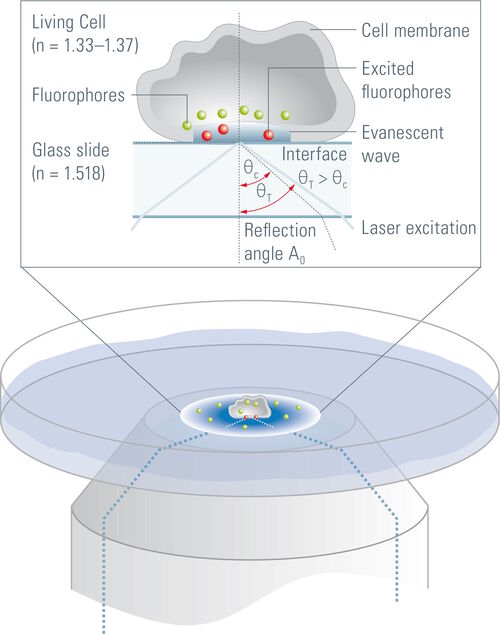
It allows imaging of fluorescent molecules located close to the glass/water (or glass/specimen) interface. This is achieved by employing an evanescent wave for excitation of the fluorophores instead of direct illumination via light delivered by an arc lamp, LEDs or lasers. The evanescent field occurs if incident light is totally reflected at the interface of two transparent media with different refractive indices. In biological applications the incident light is usually laser light and the interface the glass of the coverslip and a film of aqueous solution between coverslip and adherent cells.
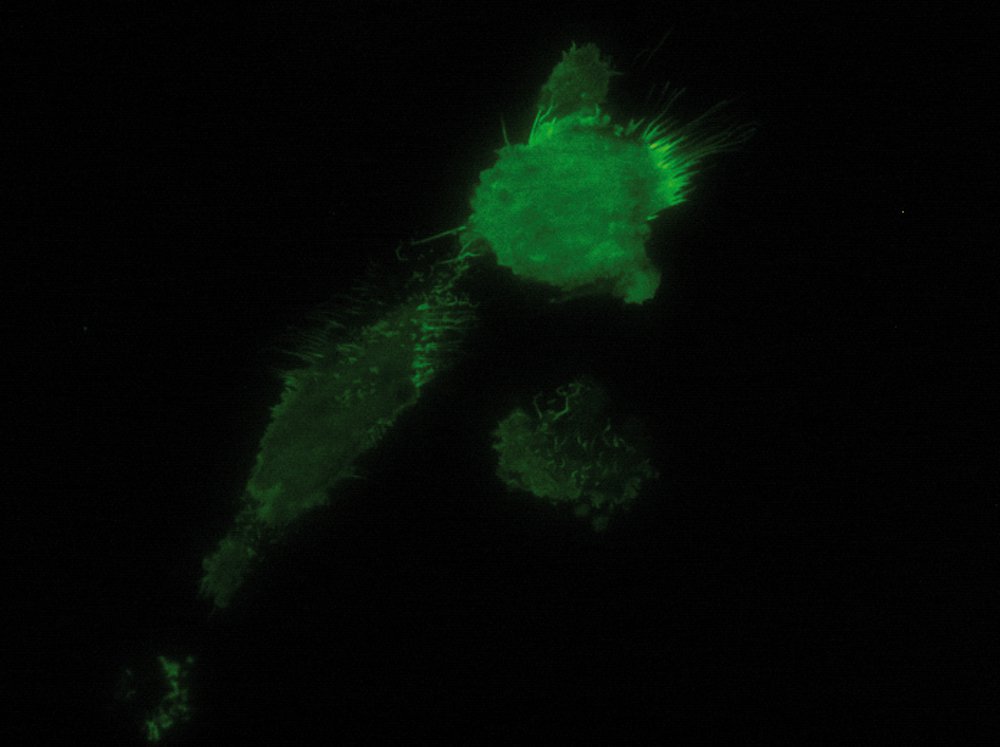
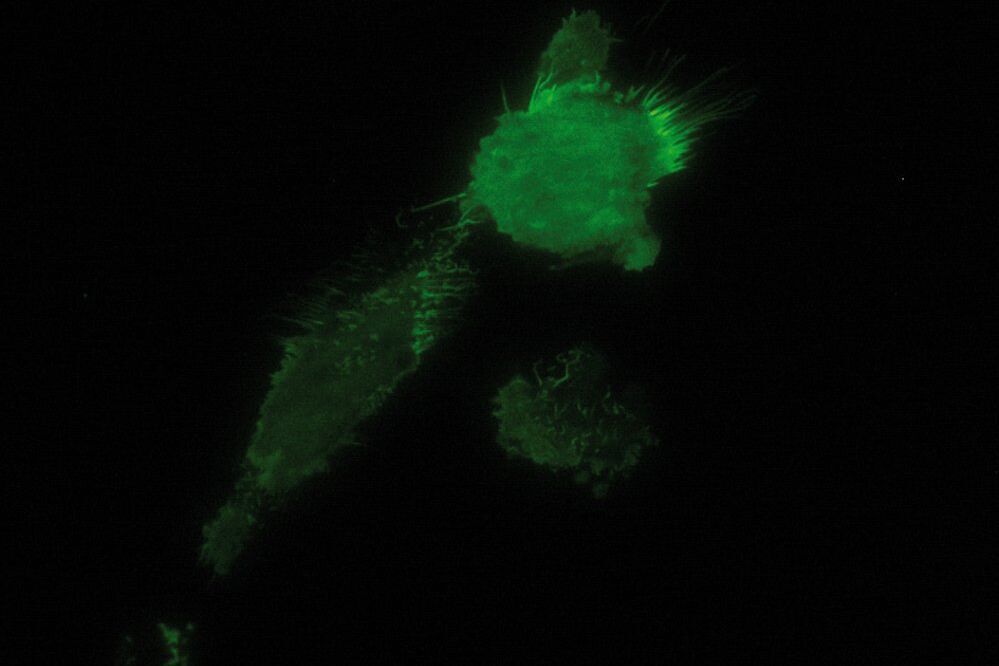
As the energy of an evanescent field decreases exponentially with distance to the interface, only fluorophores in a certain proximity to the coverslip are excited. This allows the creation of images with outstanding signal-to-noise ratio, as fluorophores in the rest of the cell are hardly excited. Additionally, TIRF microscopy delivers images with an outstandingly high axial resolution below 100 nm. This allows the observation of membrane-associated processes like cell adhesion, hormone binding, molecule transport and exocytotic and endocytotic processes (e.g. neurotransmitter release and uptake).
Total internal reflection and the evanescent field
Whenever light encounters the interface of two transparent media with different refractive indices, it will be partially diffracted and partially reflected. At a certain angle of incidence, the so called critical angle, the light will be completely reflected and a phenomenon called total internal reflection occurs. Total internal reflection can only be observed if the light travels from a medium with a higher refractive index (n) (e.g. glass dish, n = 1.52) to a medium with a lower refractive index (e.g. aqueous medium, n = 1.33). The critical angle (ΘC) of incident light, at which total internal reflection occurs, can be determined by Snell’s law:
On occurrence of total internal reflection, a portion of the energy of the incident light will be converted to an electromagnetic field and pass through the interface to form an evanescent wave originating at the interface. The emerging evanescent wave has the same frequency as the incident light and its amplitude decays exponentially with depth of penetration. Hence, fluorophores within the evanescent wave are not excited by the interaction with photons but by the interaction with the electromagnetic field. The penetration depth of this field typically ranges from 60 to 100 nm but can go up to 200 nm. It depends on the light’s angle of incidence, wavelength, and the refractive indices of the two media (e.g. the glass of the coverslip and the specimen). Increasing the angle of incidence of the light leads to a reduced penetration depth and a higher wavelength of the incident light leads to an increased penetration depth. The refractive index of the medium behind the interface (e.g. the specimen) also has an influence on the penetration depth, as a higher refractive index increases the evanescent wave’s penetration depth. It should also be stated that in TIRF microscopy high power laser light, which is stronger than the laser light usually employed in confocal systems, is used to form an evanescent wave with sufficient energy.
Objectives of a TIRF microscope
There are two approaches for achieving total internal reflection in optics: one is prism-based and the other objective-based. In prism-based TIRF microscopy, a prism is attached to the coverslip’s surface which directs a focused light beam or laser towards the coverslip/medium interface. With the help of the prism the angle of the penetrating light is adjusted to the critical angle.
Modern TIRF microscopy systems are usually objective-based. The light, usually laser light, is directed to the specimen through the objective, which also collects the emitted fluorescence light. It is mandatory that objectives for TIRF microscopy feature an extremely high numerical aperture (NA) (> 1.45 NA) which allows an angle of incidence greater than the critical angle. The higher the NA of the objective, the lower the possible penetration depth of the evanescent field, as the angle of incidence of the light can be more flat. Compared to the prism type method, the objective lens method is more convenient to use as the specimen is well accessible and the angle of incidence of the laser light can be changed easily. By placing the laser spot in different areas in the back focal plane of the objective, the user can choose the angle of incidence of the laser light and therefore change the penetration depth of the evanescent wave. In prism-based TIRF microscopy systems the prism strongly limits the access to the specimen and makes it difficult to e.g. change media, add drugs or carry out physiological measurements.
Also, the alignment of the laser and using different angles of incidence is much more complicated in prism-based systems. Moreover, laser safety concerns that arise in a prism-based TIRF system are overcome by objective-based systems. While the laser in prism-based systems is more or less openly guided into the prism, the laser in objective-based systems is directly coupled into the microscope itself and exits the objective in a very defined manner.
Total internal reflection in a biological setup
As stated above, TIRF microscopy objectives feature a high numerical aperture (> 1.45 NA), which can only be achieved by using immersion oil or other specialized liquid immersion media. In a typical biological setup, the laser light used for producing the evanescent wave has to pass through the objective, the immersion medium and the coverslip to be reflected at the coverslip/water (e.g. aqueous Ringer’s solution, PBS or other imaging buffer) interface. To avoid reflection and deflection effects, the refractive index of the immersion medium has to be as close as possible to that of the coverslip (typically n = 1.52). For standard immersion oils the refractive index is n= 1.515 – 1.518 at 20 °C. Of course, the refractive index of the immersion medium is temperature-dependent. As many experiments, especially in live cell imaging, are conducted at 37 °C, temperature-induced changes in the refractive index of the immersion medium can occur. To correct for these changes, many TIRF microscopy objectives are equipped with a correction collar.
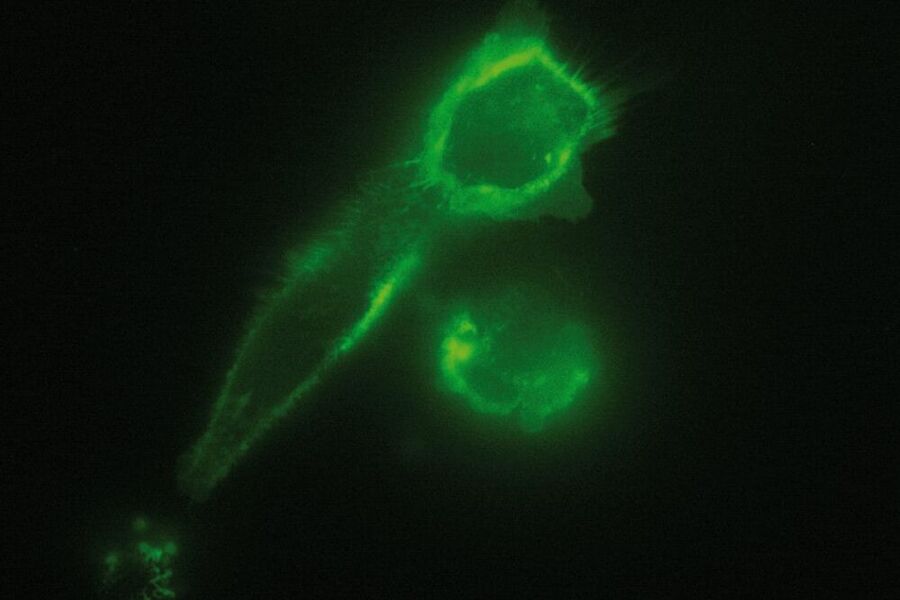
In a biological setup, the interface at which total reflection occurs is usually that between the glass coverslip with n = 1.52 and the small film of aqueous medium between coverslip and cell (n = 1.33). The evanescent field then passes through this aqueous film, the plasma membrane with a diameter of approximately 7.5 nm and proceeds to the cytosol of the cell where it declines to zero at a certain penetration depth which depends on the angle of incidence of the laser light. In some spots the cells are directly adhered to the coverslip and no aqueous solution can be found between the coverslip and the cell (e.g. at focal adhesions of cells). In this case the interface between coverslip and cell is the place of total internal reflection. As cells have a different refractive index (approximately n = 1.38) than aqueous solution, these spots might not be visible when the system is set up for the interface of coverslip/aqueous solution. According to Snell’s law, the critical angle where total reflection occurs depends on the refractive indices of the media and is therefore not the same for the interface of coverslip/aqueous solution as for the coverslip/cell interface. This problem can be overcome by changing the angle of incidence of the laser light .
As it is in the nature of the evanescent field to decline exponentially, only fluorophores in a range of approximately 60–100 nm (depending on the settings) to the interface of coverslip/aqueous solution (or the cell itself) are excited. This provides an axial resolution (in z-plane) of typically 60–100 nm, enabling the observation of fluorophores that are located in or close to the plasma membrane without being overwhelmed by fluorescence from fluorophores located in the rest of the cell. The fact that only fluorophores in a "slice" of the cell are excited by the evanescent field leads to the production of images with a very good signal-to-noise ratio with hardly any background fluorescence from out-of-focus planes.